Fig. 2.
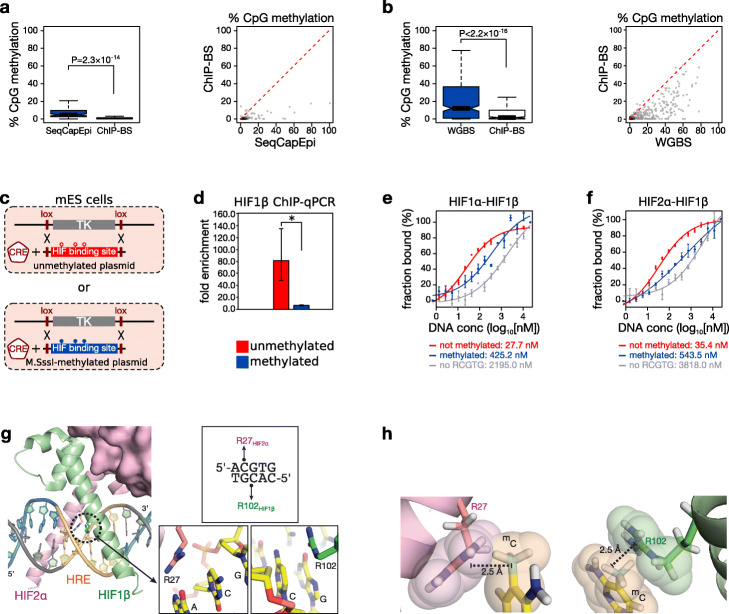
DNA methylation directly repels HIF1β binding. a, b Boxplot (left) and scatter plot (right) of methylation levels of HIF1β-bound immunoprecipitated DNA fragments obtained by ChIP-BS-seq (ChIP-BS) compared to input by SeqCapEpi BS-seq (SeqCapEpi) in MCF7 cells (a), or of HIF1β-bound immunoprecipitated DNA fragments obtained by ChIP-BS compared to input by whole-genome BS-seq (WGBS) in mouse Tet-triple-knockout (Tet-TKO) ESCs (b). The red dotted line in the scatter plot indicates the theoretical value of equal methylation in immunoprecipitated and input DNA. P values by t-test. c, d Recombination-mediated cassette exchange. c A human HIF binding site (chr16: 30,065,212-30,065,711 on hg38) was cloned between 2 L1 Lox sites and in vitro methylated (blue) or not (red) prior to co-transfection with a CRE recombinase-encoding plasmid into mESCs transformed to contain an L1 Lox-flanked thymidine kinase (TK). d Following successful cassette exchange, these ESCs were incubated in hypoxia (0.5% O2 for 16 h) and probed using HIF1β ChIP-qPCR for HIF binding at the differentially methylated cassette. Shown is the fold enrichment over background (n = 3 independent ChIP pairs; *P < 0.05 by t-test). e, f Microscale thermophoresis-based assessment of sensitivity of HIF1α-HIF1β (e) and HIF2α-HIF1β (f) heteroduplexes to methylation at HIF binding sites in physiological buffer (PBS). RCGTG sequences in the double-stranded DNA oligonucleotides were either absent (gray), methylated (blue), or unmethylated (red) at the CpG site. Calculated KD values are shown under each graph. g Excerpt from the crystal structure of HIF2α-HIF1β in complex with a DNA duplex containing the core HIF binding sequence 5′-ACGTG-3′ (PDB code 4ZPK) [25]. h Modeling of methylation of CpG cytosines in ACGTG reveals severe steric hindrance. The two views show hard-sphere models of methylated cytosines modeled at position 5 (including bonding hydrogen atoms) and how they severely violate the van der Waals envelopes (2.5 Å width) of Arg27 in HIF2α (left) and Arg102 in HIF1β (right)