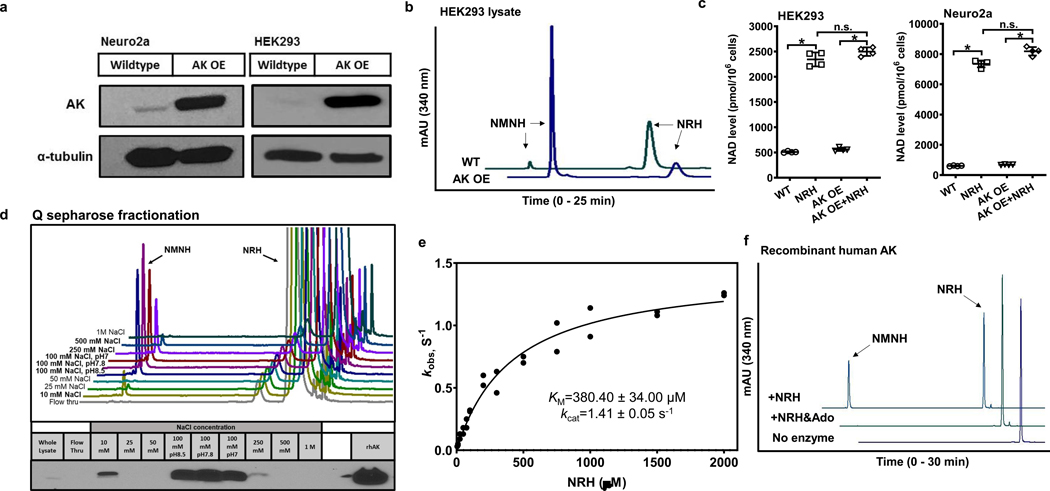
Figure 3.
Characterization of AK as an NRH kinase. a) Western blot showing overexpression of human AK in Neuro2a and HEK293 cells with pcDNA3.1 AK vector. b) AK overexpressing lysate in HEK293 cells produces more NMNH than wildtype HEK293 lysate. Experiments were repeated independently 3 times with similar results. c) AK overexpression in HEK293 and Neuro2a cells treated with 1 mM NRH for 6 hr. Data expressed as mean ± SD, n=4 biologically independent samples. In b) & c), one-way ANOVA and Tukey’s multiple comparison test were used for statistical analysis, * indicates p<0.0001; n.s. indicates p>0.05. d) AK overexpressing cell lysate in HEK293 cells have NRH kinase active fractions co-eluting with AK after Q sepharose separation. Bottom panel shows western blots for AK in different fractions. Experiment was repeated independently 2 times with similar results. e) Kinetic parameters of recombinant human AK enzyme with NRH as a substrate. n=2 independent experiments. Data is plotted and fit to Michaelis-Menton equation. f) Substrate competition test of AK with NRH and Adenosine (Ado). Addition of 2 mM Ado in the reaction inhibited the NMNH production from 1 mM NRH using 0.5 ug recombinant human AK, with 2 mM ATP/Mg2+ in pH 7.4 phosphate buffer. Experiments were repeated independently 2 times with similar results.