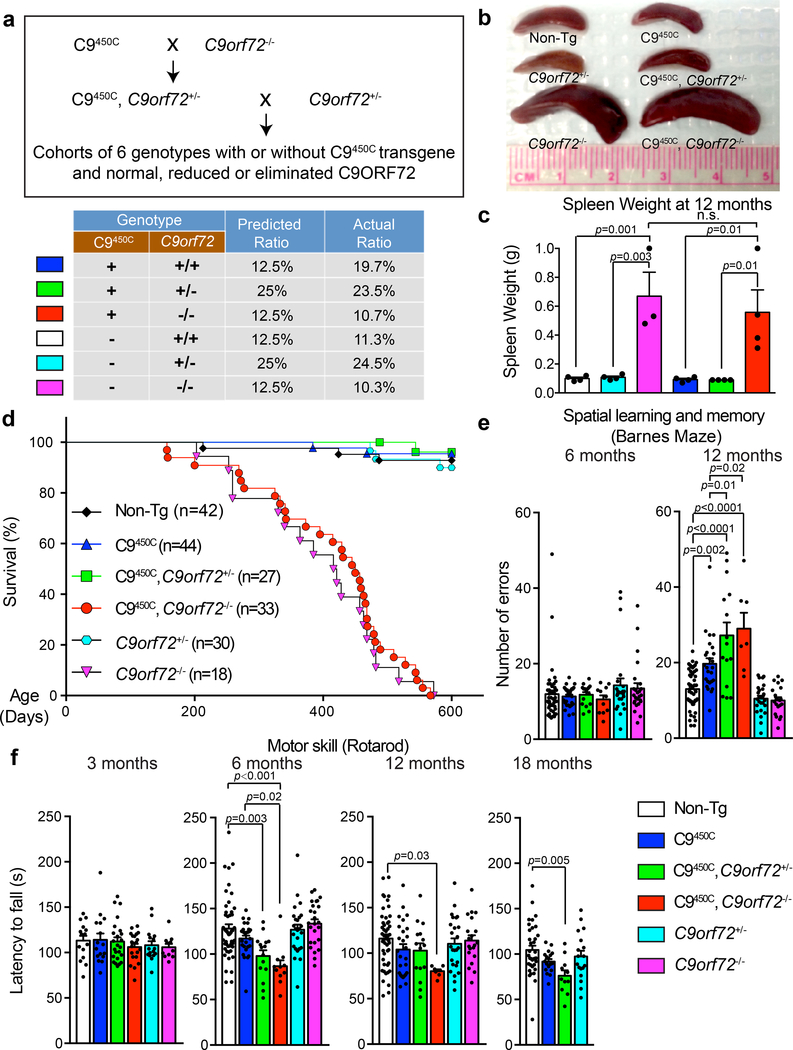
Figure 3: Reduction or loss of endogenous C9ORF72 exacerbates age-dependent cognitive abnormalities and motor deficits in C9450C mice.
(a) Schematic of the breeding strategy to produce C9orf72 transgenic mice with normal, reduced or absence of endogenous C9ORF72, including the predicted ratios for Mendelian inheritance of each genotype and observed ratios in the breeding cohorts.
(b) Spleen sizes at 12 months of age for mice with indicated genotypes. Experiment was reproduced three times independently with similar results.
(c) Spleen weight at 12 months of age. Error bars represent SEM (n = 4 Non-Tg, n = 4 C9orf72+/−, n = 3 C9orf72−/−, n = 4 C9450C, n = 4 C9450C,C9orf72+/−, n = 4 C9450C,C9orf72−/−). Statistical significance assessed with one-way ANOVA with Tukey’s post hoc test. n.s., not significant.
(d) Survival curve up to 600 days for mice with indicated genotypes.
(e-f) Behavioral performance in C9orf72 transgenic mice with normal, reduced or absence of C9ORF72 (n = 49 [Non-Tg], n = 28 C9450C, n = 15 C9450C,C9orf72+/−, n = 11 C9450C,C9orf72−/−, n = 25 C9orf72+/−, n = 25 C9orf72−/−). (e) Spatial learning and memory performance on a Barnes maze at 6 and 12 months of age showing the number of errors in finding the escape chamber at days 7–9. (f) Motor performance on a rotarod measured by the latency to fall at 6, 12 and 18 months of age. A separate cohort of C9orf72 transgenic mice with normal, reduced, or absence of endogenous C9ORF72 (n = 15 Non-Tg, n = 16 C9450C, n = 24 C9450C,C9orf72+/−, n = 21 C9450C,C9orf72−/−, n = 16 C9orf72+/−, n = 11 C9orf72−/−) was tested at 3 months of age. Error bars represent SEM. Statistical evaluations were performed using one-way ANOVA with Tukey’s post hoc test.