Figure: Study selection.
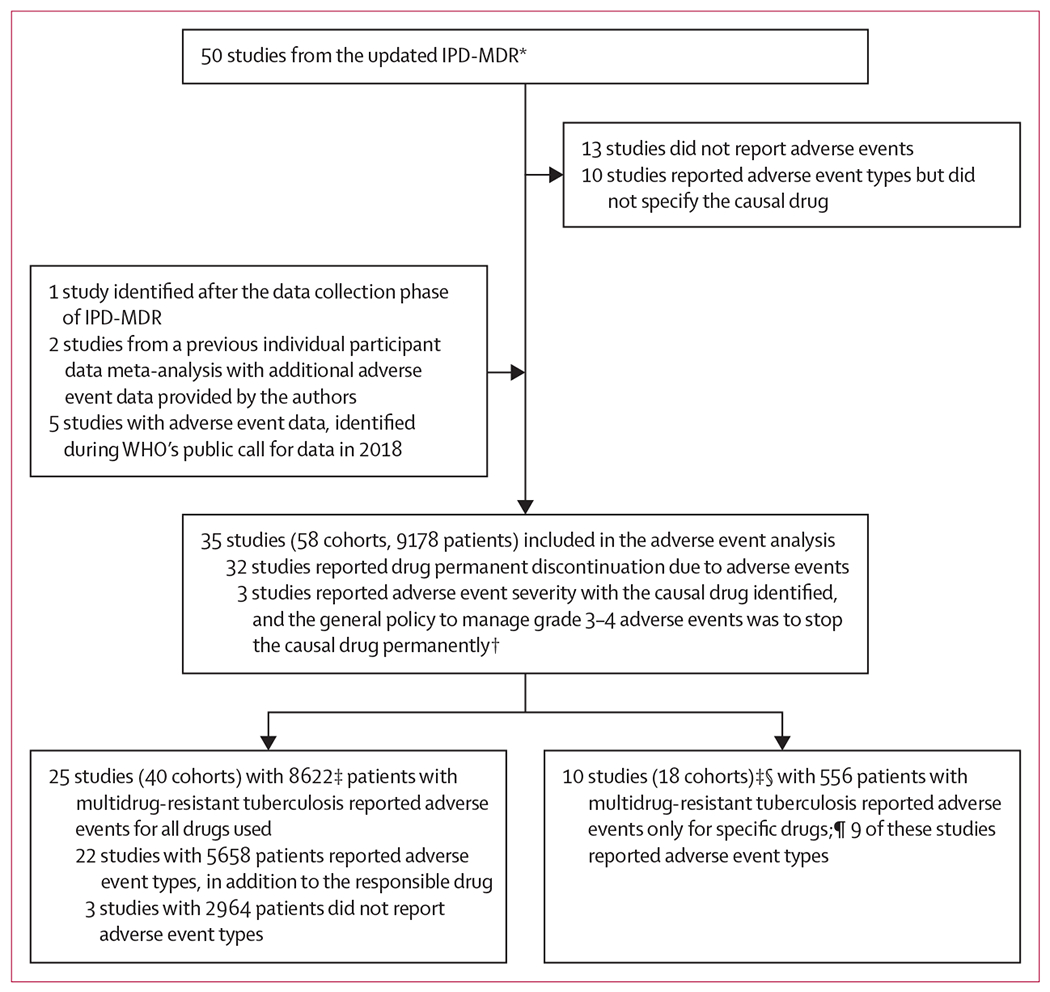
In all analyses, adverse events were defined as those that resulted in permanent discontinuation of a drug. IPD-MDR=individual patient data meta-analysis for multidrug-resistant tuberculosis. *For details of the selection of the 50 studies, refer to Ahmad et al (2018).14 †For these three studies, if the grade of an adverse event was 3–4, the causal drug was considered as permanently stopped. ‡Patients without treatment regimen information and patients who were still on treatment were excluded, patients with extrapulmonary disease were included. §Two studies had adverse event information for more than one drug. ¶dverse events for bedaquiline reported: one study with 130 patients; adverse events for linezolid reported: ten studies with 508 patients; adverse events for carbapenem reported: two studies with 139 patients (each patient number includes only the patients who used the drug for which adverse events were reported).
