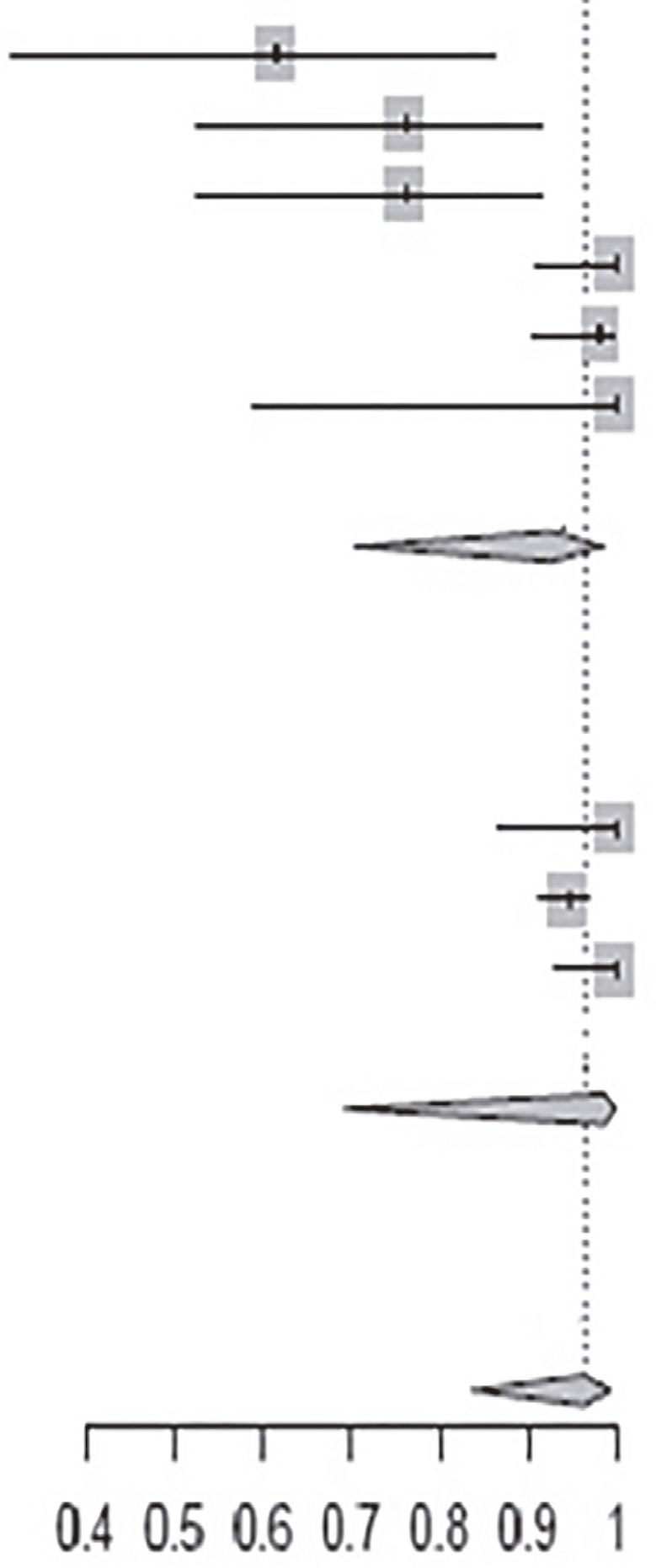
Table 3. Evolution of PCR parameters, cardiac conditions and serology among studies on benznidazole treatment for 30 and 60 days.
| Study | Events | Total | Proportion 95% CI | |
|---|---|---|---|---|
|
| ||||
| Time of treatment: 60 days | ||||
| Andrade et al.12 | 8 | 13 | 0.62 [0.32; 0.86] |
|
| Andrade et al.13 | 16 | 21 | 0.76 [0.53; 0.92] | |
| Oliveira et al.16 | 16 | 21 | 0.76 [0.53; 0.92] | |
| Pérez-Antón et al.19 | 38 | 38 | 1.0 [0.91; 1.00] | |
| Pinazo et al.29 | 55 | 56 | 0.98 [0.90; 1.00] | |
| Vallejo et al.33 | 7 | 7 | 1.0 [0.50; 1.00] | |
| Subtotal (95% CI) | 156 | 0.93 [0.71; 0.99] | ||
| Total Events | 140 | |||
| Heterogeneity: I2 = 84%, π2 = 2.8331, p = 0.09 | ||||
| Time of treatment: 30 days | ||||
| Coura et al.22 | 26 | 26 | 1.00 [0.87; 1.00] | |
| Viotti et al.31 | 268 | 283 | 0.95 [0.91; 0.97] | |
| Viotti et al.32 | 53 | 53 | 1.00 [0.93; 1.00] | |
| Subtotal (95% CI) | 362 | 0.99 [0.69; 1.00] | ||
| Total Events | 347 | |||
| Heterogeneity: I2 = 60%, π2 = 1.6063, p = 1.00 | ||||
| Total (95% CI) | 518 | 0.97 [0.84; 0.99] | ||
| Total Events | 487 | |||
| Heterogeneity: I2 = 90%, π2 = 3.8301, p < 0.01 | ||||
| Residual heterogeneity: I2 = 25%, p = 0.23 | ||||
