Abstract
Polymerase chain reaction (PCR)-based molecular markers have been developed to detect the presence of primary parasitoids in cereal aphids and used to estimate primary parasitism rates. However, the presence of secondary parasitoids (hyperparasitoids) may lead to underestimates of primary parasitism rates based on PCR markers. This is because even though they kill the primary parasitoid, it’s DNA can still be amplified, leading to an erroneous interpretation of a positive result. Another issue with secondary parasitoids is that adults are extremely difficult to identify using morphological characters. Therefore, we developed species-specific molecular markers to detect hyperparasitoids. A 16S ribosomal RNA mitochondrial gene fragment was amplified by PCR and sequenced from two secondary parasitoid species, Dendrocerus carpenteri (Curtis) (Hymenoptera: Megaspilidae) and Alloxysta xanthopsis (Ashmead) (Hymenoptera: Charipidae), four geographic isolates of the primary parasitoid, Lysiphlebus testaceipes (Cresson) (Hymenoptera: Braconidae), and six aphid species common to cereal crops. Species-specific PCR primers were designed for each insect on the basis of these 16S rRNA gene sequences. Amplification of template DNA, followed by agarose gel electrophoresis, successfully distinguished D. carpenteri and A. xanthopsis from all four isolates of L. testaceipes and all six cereal aphid species in this laboratory test.
Keywords: 16S RNA, aphididae, braconidae, charipidae, homoptera, hymenoptera, megaspilidae, mtDNA, PCR, primary parasitoid, secondary parasitoid
Introduction
Controlling cereal aphids (Homoptera: Aphididae) with synthetic insecticides can potentially lead to insecticide-resistance in aphids (Teetes et al., 1975; Rider et al., 1998), can adversely impact beneficial organisms (Basedow et al., 1985; Matcham and Hawkes 1985), wildlife, and the environment (Flickinger et al., 1991; Daily et al., 1998), and at times even result in poor crop profitability (Duff et al., 1995). Biological control by arthropod natural enemies, especially Hymenopteran parasitoids, affords some positive alternatives to control by insecticides (Reed et al., 1991; Brewer et al., 1998; Farid et al., 1998).
Among predominant parasitoids attacking cereal aphids in North America is Lysiphlebus testaceipes (Cresson) (Hymenoptera: Braconidae) (Pike et al., 2000). This parasitoid is consistently the most abundant species attacking bird cherry-oat aphid, Rhopalosiphum padi (L.), corn leaf aphid R. maidis (Fitch) (Pike et al., 1997, K.S.P., unpublished), and greenbug, Schizaphis graminum (Rondani) (Knutson et al., 1993; Royer et al., 2001). Its overall success as a primary or aphid-attacking parasitoid, however, is influenced by the presence of secondary parasitoids (hyperparasitoids), parasitoids that utilize the primary parasitoid as a host and kill it (Starks et al., 1976; Sullivan 1988).
The species diversity and abundance of hyperparasitoids attacking aphidiine parasitoids, such as L. testaceipes, can be relatively high at times. Several genera of aphid-associated hymenopteran hyperparasitoids have been reported: Asaphes, Coruna, and Pachyneuron (Pteromalidae); Dendrocerus (Megaspilidae); Alloxysta, Lytoxysta, and Phaenoglyphis (Charipidae: Alloxystinae); and Aphidencyrtus (Encyrtidae) (Bennett and Sullivan, 1978; Bocchino and Sullivan, 1981; Matejko and Sullivan, 1984; Carew and Sullivan 1993; Gibson et al., 1997; Pike et al., 1997; Al Dobai et al., 1999). One species, Dendrocerus carpenteri (Curtis), has been reported as a major hyperparasitoid of L. testaceipes (Scholz and Höller, 1992; Sullivan and Völkl, 1999) and widely distributed in the world, including North America, Europe, Japan, Australia, and New Zealand. Alloxysta xanthopsis (Ashmead) is another species commonly reared from L. testaceipes, but its range is limited to North America including Florida, Alaska, British Columbia, California, Utah (Andrews, 1978), and Washington (K.S.P. unpublished). Studies of secondary parasitoids are confounded by the difficulty in identification of adults using morphological characters. Even identification to family can be problematic.
Molecular markers based on the polymerase chain reaction (PCR) have been developed for use in detecting primary parasitoids in aphids (Zhu et al., 2000; Weathersbee et al., 2004; Jones et al., 2005). While the method employed by Jones et al. (2005) accurately estimated the parasitism level of the primary parasitoid L. testaceipes in greenbug populations on wheat, the test was done in November when hyperparasitoid populations are relatively low. During the summer months, hyperparasitism is a common occurrence in the sorghum-aphid-L. testaceipes system in the Southern Great Plains (K.A.S. personal observations). It is therefore likely that estimates of primary parasitism rates in sorghum (based on PCR methods) may be complicated by hyperparasitoids.
Our research objectives were twofold. First, to develop species-specific PCR primers for the hyperparasitoids of the primary parasitoid, L. testaceipes, and second, to demonstrate that such primers could be used to differentiate hyperparasitoid DNA from that of L. testaceipes and six species of cereal aphids. Our research focused on the use of the 16S ribosomal RNA gene (16S rDNA). As a mitochondrial gene, it occurs as multiple copies per cell, which increases the likelihood of successful amplification in gut extracts. It also offers various levels of variability (Zhang and Hewitt, 1996), allowing closely related species to be separated (Chen et al., 2002).
Materials and methods
Insects
Aphids, from laboratory colonies maintained at the USDA-ARS research unit in Stillwater, Oklahoma, were used. We chose six common species of the U.S. Great Plains cereal aphid (Homoptera: Aphididae) complex: R. padi; R. maidis; English grain aphid, Sitobion avenae (F.); S. graminum; Russian wheat aphid, Diuraphis noxia (Kurdjumov); and yellow sugarcane aphid, Sipha flava (Forbes).
Lysiphlebus testaceipes were collected from Payne County, Oklahoma (via adult emergence from R. maidis, R. padi and S. graminum mummies found on sorghum) and from Whitman, Chelan, and Kittitas Counties, Washington. Dendrocerus carpenteri were reared from Aphis helianthi Monell (on Gray’s lovage, Ligusticum grayi) through the primary parasitoids L. testaceipes (main host) and an Ephedrus sp. The hyperparasitoid, Alloxysta xanthopsis (Ashmead) was reared from the milkweed aphid, Aphis nerii Boyer de Fonscolombe (on milkweed, Asclepias speciosa) through the primary parasitoid, L. testaceipes. All hyperparasitoid rearing was done at Prosser, Washington and identifications were made by K.S.P. using Andrews (1978) and Dessart (1972). Voucher specimens are held in the Washington State University, Irrigated Agriculture Research and Extension Center, 24106 N Bunn Rd., Prosser, WA 99350.
DNA extraction and PCR amplification
Genomic DNA was isolated from individual wasps, without regard to sex, as previously described by Chen et al. (2000). Single insects were placed in 1.5-ml microcentrifuge tubes and homogenized using a battery-powered homogenizer (Midwest Scientific, St. Louis, Missouri) in 100 μl of isolation buffer containing 0.1 M NaCl, 0.2 M sucrose, 0.1 M Tris–HCl (pH 9.1), 0.05 M EDTA, 1% SDS, and 20 μg/ml RNase A. The homogenate was vortexed briefly and incubated for 30 min at 65 °C. The solution was transferred to a new tube and extracted once with one volume of chloroform/isoamyl alcohol (24:1). DNA was precipitated by adding one-tenth volume of 3.0 M sodium acetate and two volumes of ice-cold 100% ethanol, and then centrifuged after 30 min. The DNA pellet was then air dried and suspended in 200 μl distilled water.
PCR amplification, purification, and sequencing of 16S rDNA
Portions of the mitochondrial 16S rRNA gene were PCR amplified after Chen et al. (2002). The primers used were 16SF or 16SWb (5′-CACCTGTTTATCAAAAACAT-3′), which anneals to nucleotides 13924–13943, and 16SR1 or 16SWa (5′-CGTCGATTTGAACTCAAATC-3′), which anneals to nucleotides 13392–13411 of the honey bee mitochondrial genome (Crozier and Crozier, 1993). Where amplifications were unsuccessful under a variety of conditions, the primer 16SR2 or 16S.Sh (5′-AGATTTTAAAAGTCGAACAG-3′), which anneals to nucleotides 13417–13436 of the honey bee genome, was used in place of 16SWa. The PCR product from the 16S rRNA gene was thus in the range of 530–550 bp.
PCR reactions (25 μl) contained 10 mM Tris--HCl pH 9.0, 1.5 mM MgCl2, 1.0 μM of each primer, 50 mM KCl, 0.1 mM of each dNTP, 0.05 U/μl of Taq DNA polymerase (Promega, Madison, Wisconsin), and 2 μl of DNA template solution, and were performed in a PTC-100 Thermal Controller (MJ Research, Watertown, Massachusetts). Template DNA was denatured for 3 min at 94 °C, followed by 35 amplification cycles, with 30 s denaturing at 94 °C, 30 s annealing at 50 °C, and 1 min extension at 72 °C. DNA was finally extended for 2 min at 72 °C after amplification.
PCR products were separated on a 1% low melting point agarose gel. DNA fragments were excised from the gel and extracted using a Wizard PCR Preps DNA Purification System (Promega). Purified PCR products were directly sequenced using an automated sequencer located at the Recombinant DNA/Protein Resource Facility, Oklahoma State University, Stillwater, Oklahoma and at the DNA Core facility, Marshall University, Huntington, West Virginia. All insects were sequenced using above stated primers, except A. xanthopsis in which a pair of internal primers were also designed and used to attain the complete sequence; DcarpF (5′-GTAAACTGGAATGAATGATTTAATA-3′) and AlloxR (5′-CTTAATTCAACATCGAGGTC-3′).
Primer design and PCR amplification of aphid and parasitoid DNA
After obtaining all aphid, parasitoid, and hyperparasitoid 16S rDNA sequences, we used GCG Wisconsin Package UNIX Version 10 (Genetics Computer Group, Madison, Wisconsin, USA) for alignment and analysis. Primers were designed to separate D. carpenteri from L. testaceipes and all six cereal aphids. We used the single base detection technique (Kwok et al., 1990) to design primers for the detection of the hyperparasitoids. PCR reactions (25 μl) contained 10 mM Tris–HCl pH 9.0, 1.5 mM MgCl2, 1.0 μM of each primer, 50 mM KCl, 0.1 mM of each dNTP, 0.05 U/μl of Taq DNA polymerase (Promega), and 2 μl of template containing 10–100 ng DNA. For the PCR reaction, DNA was denatured for 3 min at 94 °C, followed by 35 amplification cycles, with 30 s denaturing at 94 °C, 30 s annealing at 57 °C (Innis et al., 1990), and 1 min extension at 72 °C. DNA was finally extended for 2 min at 72 °C after amplification. PCR products were separated on a 1.5% agarose gel, stained with ethidium bromide, and photographed under ultraviolet light.
Sensitivity
We determined the sensitivity for PCR detection of hyperparasitoid DNA by subjecting serial 10-fold dilutions of D. carpenteri DNA, in the standard dilution of extracted DNA of R. padi, and L. testaceipes (10−2 insect equivalents of L. testaceipes and R. padi).
Results and discussion
All DNA sequences were submitted to GenBank with the accession numbers: AY745772--AY745781. Sequences were confirmed as 16S rDNA by similarity searching the GenBank Blastn and Fasta database. Both 16SR1 and 16SR2 are located on the 3′ downstream end of the 16S rRNA gene, while 16SF is located on the 5′ upstream end. These sequences are highly conserved, and the corresponding primers have worked for many hymenopteran species (Dowton and Austin 1994, 1997). In our experiment, the primer pairs amplified all species and populations of wasps and aphids. No species-specificity was observed for the primer pairs.
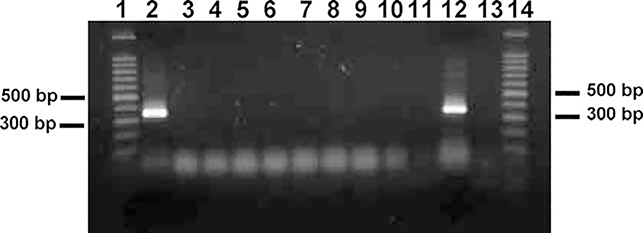
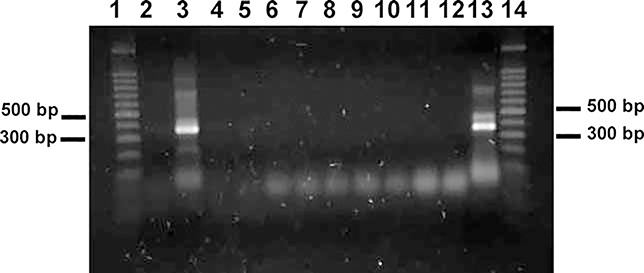
From the known 16S rDNA sequences, DcarpF (see above for sequence) and DcarpR (5′-TCGAGGTCGCAAATAACTTA-3′), a pair of species-specific primers were designed to separate D. carpenteri from A. xanthopsis, L. testaceipes, and all six aphid species. DcarpF and AlloR1 (5′-CATCGAGGTCATAATCTTTTTTATCA-3′) were designed to separate A. xanthopsis from D. carpenteri, L. testaceipes, and all six aphid species. The DcarpF and DcarpR primer pair successfully amplified a 341 bp fragment for D. carpenteri (Figure 1). The DcarpF and AlloR1 primer pair successfully amplified a 335 bp fragment from A. xanthopsis (Figure 2). In neither case was L. testaceipes or any aphid template DNA amplified.
Figure 1.
PCR amplification of Dendrocerus carpenteri DNA using primers DcarpF and DcarpR yielded a 341 bp fragment. Lanes 1 and 14, 100 bp DNA ladder; Lane 2, D. carpenteri; Lane 3, Alloxysta xanthopsis from Prosser, WA; Lane 4, Lysiphlebus testaceipes from Whitman County, Washington; Lane 5, L. testaceipes from Payne County, Oklahoma; Lane 6, Schizaphis graminum; Lane 7, Diuraphis noxia; Lane 8, Rhopalosiphum padi; Lane 9, Sipha flava; Lane 10, R. maidis; Lane 11, Sitobion avenae; Lane 12, DNA mix of D. carpenteri, L. testaceipes and R. padi; Lane 13, DNA mix of A. xanthopsis, L. testaceipes and R. padi.
Figure 2.
PCR amplification of Alloxysta xanthopsis DNA using primers DcarpF and AlloR1 yielded a 335 bp fragment. Lanes 1 and 14, 100 bp DNA ladder; Lane 2, Dendrocerus carpenteri; Lane 3, A. xanthopsis from Prosser, WA; Lane 4, Lysiphlebus testaceipes from Whitman County, Washington; Lane 5, L. testaceipes from Payne County, Oklahoma; Lane 6, Schizaphis graminum; Lane 7, Diuraphis noxia; Lane 8, Rhopalosiphum padi; Lane 9, Sipha flava; Lane 10, R. maidis; Lane 11, Sitobion avenae; Lane 12, DNA mix of D. carpenteri, L. testaceipes and R. padi; Lane 13, DNA mix of A. xanthopsis, L. testaceipes and R. padi.
Due to the difficulty in rearing hyperparasitoids under laboratory conditions, we simulated a hyperparasitized host by extracting DNA from a single individual of D. carpenteri, L. testaceipes, and R. padi, respectively, then mixed all three DNA solutions together. The ratio of DNA concentrations of the above three species was 1:1:1 in terms of insect equivalents. The same approaches were used to mix DNAs of A. xanthopsis, L. testaceipes and R. padi. The DNA mixture was then subjected to PCR, and a 341 bp fragment was amplified demonstrating a positive detection of D. carpenteri and a negative detection of A. xanthopsis (Figure 1). For the Dcarp and AlloR1 primer pair, only a 335 bp fragment was amplified demonstrating a positive detection of A. xanthopsis and a negative detection of D. carpenteri (Figure 2). Therefore, the species specific primers for detecting D. carpenteri were DcarpF (5′-GTAAACTGGAATGAATGATTTAATA-3′) and DcarpR (5′-TCGAGGTCGCAAATAACTTA-3′), and the species specific primers for detecting A. xanthopsis were DcarpF (5′-GTAAACTGGAATGAATGATTTAATA-3′) and AlloR1 (5′-CATCGAGGTCATAATCTTTTTTATCA-3′).
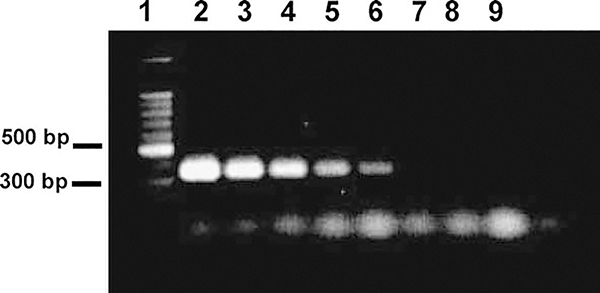
The threshold sensitivity of PCR to detect D. carpenteri DNA in a mixture of L. testaceipes and R. padi DNA was 10−7 D. carpenteri adult equivalents (Figure 3). Only strongly amplified PCR products (341 bp) were observed when the amount of hyperparasitoid template DNA equaled or exceeded 10−6 adult equivalents. The molecular markers that were developed in our previous studies using this technique have been successful in detecting adult (Prinsloo et al., 2002) and immature parasitoids (Jones et al., 2005). However, they were not successful in detecting parasitoid eggs in the hosts. It is unlikely that the primers described herein would detect the egg stage of secondary parasitoids.
Figure 3.
Sensitivity of PCR detection of hyperparasitoids using primers DcarpF and DcarpR, as determined by titration of Dendrocerus carpenteri DNA. The concentration of Lysiphlebus testaceipes and Rhopalosiphum padi DNA was held constant at 10−2 host equivalents. Lane 1, 100 bp DNA ladder; Lane 2, 10−2 equivalents of D. carpenteri DNA; Lane 3, 10−3 equivalents of D. carpenteri DNA; Lane 4, 10−4 equivalents of D. carpenteri DNA; Lane 5, 10−5 equivalents of D. carpenteri DNA; Lane 6, 10−6 equivalents of D. carpenteri DNA; Lane 7, 10−7 equivalents of D. carpenteri DNA; Lane 8, 10−8 equivalents of D. carpenteri DNA; Lane 9, 10−9 equivalents of D. carpenteri DNA.
Detection of aphid parasitism is somewhat challenging because of the small size of both host and parasitoid (Greenstone, 2003). A variety of molecular methods have been used to detect and identify aphid primary parasitoids, including enzyme electrophoresis (Castañera et al., 1985; Walton et al., 1990), RAPD (Black et al., 1992; Kazmer et al., 1995) and specific PCR using ITS2 DNA (Zhu and Greenstone, 1999; Zhu et al., 2000), 16S rDNA (Prinsloo et al., 2002), and restriction enzyme digestion (Prinsloo et al., 2002). The detection and identification of hyperparasitoids is even more difficult due to their minute size. Our study is among the first to describe molecular markers to detect hyperparasitoids in primary parasitoids developing in aphids.
There was no or little variation in the 16S rDNA sequence of L. testaceipes from different populations. The sequence divergence from different populations ranges from 0 to 0.38% (Chen et al., 2002). However, 16S rDNA may be highly variable among different populations of D. carpenteri. We found only 90.5% sequence similarity between individuals of D. carpenteri from Washington State and D. carpenteri from Australia, the latter sequenced by Dowton and Austin (2001). The unusually high sequence divergence among these two populations might be the result of adaptive radiation on different hosts species or habitats.
Hyperparasitoids can negatively impact the effectiveness of the primary parasitoid, L. testaceipes (Wright and James, 2001). Accurate detection and identification of hyperparasitoids in aphids will enable one to accurately assess the impact of L. testaceipes on cereal aphids, and to achieve a better understanding of the multi-trophic interactions among aphids, primary parasitoids, and secondary parasitoids. The tools presented herein will aid studies of molecular ecology, detection and identification of two common secondary parasitoids impacting L. testaceipes and a complex of common host aphid species.
Acknowledgements
We thank Kevin Roush and Angie Sitterly for technical assistance. Valuable comments and criticisms were provided by Richard Shukle, Allen Szalanski, and Yu Cheng Zhu. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Abbreviations
- 16S rDNA
16S ribosomal RNA gene
- EDTA
ethylenediaminetetraacetic acid
- ITS2
internal transcribed spacer region number 2
- mtDNA
mitochondrial DNA
- PCR
polymerase chain reaction
- rDNA
ribosomal DNA
- SDS
sodium dodecyl sulfate
- USDA-ARS
United States Department of Agriculture-Agricultural Research Service
References
- Al Dobai S, Praslička J and Miština T, 1999. Parasitoids and hyperparasitoids of cereal aphids (Homoptera, Aphididae) on winter wheat in Slovakia. Biologia (Bratisl.) 54: 573–580. [Google Scholar]
- Andrews FG, 1978. Taxonomy and host specificity of Nearctic Alloxystinae with a catalog of the world species (Hymenoptera: Cynipidae). Occasional Papers in Entomology (State of California, Dept. of Food and Agriculture, Div. Of Plant Industry, Sacramento) No. 25: 128 pp. [Google Scholar]
- Basedow T, Rzehak H and Voss K, 1985. Studies on the effect of deltamethrin on the numbers of epigeal predatory arthropods in arable fields. Pestic. Sci. 16: 325–332. [Google Scholar]
- Bennett AW and Sullivan DJ, 1978. Defensive behavior against tertiary parasitism by the larva of Dendrocerus carpenteri, an aphid hyperparasitoid. J. N. Y. Entomol. Soc. 86: 153–160. [Google Scholar]
- Black CW IV, DuTeau NM, Puterka GJ, Nechols JR and Pettorini JM, 1992. Use of the random amplified polymorphic DNA polymerase chain reaction (RAPD-PCR) to detect DNA polymorphisms in aphids (Homoptera: Aphididae). Bull. Entomol. Res. 82: 151–159. [Google Scholar]
- Bocchino F and Sullivan DJ, 1981. Effects of venoms from two aphid hyperparasitoids, Asaphes lucens and Dendrocerus carpenteri (Hymenoptera: Pteromalidae and Megaspilidae) on larvae of Aphidius smithi (Hymenoptera: Aphidiidae). Can. Entomol. 113: 887–889. [Google Scholar]
- Brewer MJ, Struttmann JM, Oswald CJ II and Mornhinweg DW, 1998. Russian wheat aphid (Homoptera: Aphididae) found on field-grown barley lines varying in susceptibility to Russian wheat aphid. In: Quisenberry SS and Peairs FB (eds), Response Model for an Introduced Pest – the Russian Wheat Aphid (Homoptera: Aphididae). Thomas Say Publications in Entomology. Entomological Society of America, Lanham, MD: pp. 258–269. [Google Scholar]
- Carew WP and Sullivan DJ, 1993. Interspecific parasitism between two aphid hyperparasitoids, Dendrocerus carpenteri (Hymenoptera: Megaspilidae) and Asaphes lucens (Hymenoptera: Pteromalidae). Ann. Entomol. Soc. Am. 86: 794–798. [Google Scholar]
- Castañera P, Loxdale HD and Nowak K, 1985. Electrophoretic study of enzymes from cereal aphid populations. II. use of electrophoresis for identifying aphid parasitoids (Hymenoptera) of Sitobion avenae (F.) (Hemiptera: Aphididae). Bull. Entomol. Res. 73: 659–665. [Google Scholar]
- Chen Y, Giles KL, Payton ME and Greenstone MH, 2000. Identifying key cereal aphid predators by molecular gut analysis. Mol. Ecol. 9: 1887–1898. [DOI] [PubMed] [Google Scholar]
- Chen Y, Giles KL, Payton ME and Greenstone MH, 2002. Molecular evidence for a species complex in the genus Aphelinus (Hymenoptera: Aphelinidae), with additional data on Aphidiine phylogeny (Hymenoptera: Braconidae). Ann. Entomol. Soc. Am. 95: 29–34. [Google Scholar]
- Crozier RH and Crozier YC, 1993. The mitochondrial genome of the honeybee Apis mellifera: complete sequence and genome organization. Genetics 133: 97–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily G, Dasgupta P and Bolin B, 1998. Food production, population growth, and the environment. Science 281: 1291–1292. [DOI] [PubMed] [Google Scholar]
- Dessart P, 1972. Révision des espèces européennes du genre Dendrocerus Ratzeburg, 1852 (Hymenoptera Ceraphronoidea). Mem. Soc. R. Ent. Belg. 32: 310. [Google Scholar]
- Dowton M and Austin AD, 1994. Molecular phylogeny of the insect order Hymenoptera: apocritan relationships. Proc. Nat. Acad. Sci. USA. 91: 9911–9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowton M and Austin AD, 1997. The evolution of strand-specific compositional bias. A case study in the Hymenopteran mitochondrial 16S rRNA gene. Mol. Biol. Evol. 14: 109–112. [DOI] [PubMed] [Google Scholar]
- Dowton M and Austin AD, 2001. Simultaneous analysis of 16S, 28S, COI and morphology in the Hymenoptera: Apocrita-evolutionary transitions among parasitic wasps. Biol. J. Linn. Soc. 74: 87–111. [Google Scholar]
- Duff B, Rasmussen PE and Siley RW, 1995. Wheat/fallow systems in semi-arid regions of the Pacific NW America In: Barnett V, Payen R and Steiner R (eds), Agricultural Sustainability: Economic, Environmental and Statistical Considerations. John Wiley and Sons, New York, NY: pp. 85–110. [Google Scholar]
- Farid A, Johnson JB, Shafii B and Quisenberry SS, 1998. Tritrophic studies of Russian wheat aphid, a parasitoid, and resistant and susceptible wheat over three parasitoid generations. Biol. Control 12: 1–6. [Google Scholar]
- Flickinger EL, Juenger G, Roffe TJ, Smith MR and Irwin RJ, 1991. Poisoning of Canada geese in Texas by parathion sprayed for control of Russian wheat aphid. J. Wildl. Dis. 27: 265–268. [DOI] [PubMed] [Google Scholar]
- Gibson GAP, Huber JT and Woolley JB, 1997. Annotated Keys to the Genera of Nearctic Chalcidoidea (Hymenoptera). NRC Research Press, Ottawa. [Google Scholar]
- Greenstone MH, 2003. Assessing insect parasitism by PCR: Applications to classical biological control In: Van Driesche R (ed), Proceedings of the First International Symposium on Biological Control of Arthropods, Honolulu, HI, 14–18 January 2002. USDA Forest Service Publication FHTET-03–05; pp. 98–101. [Google Scholar]
- Innis MA, Gelfand DH, Sninsky JJ and White TJ, 1990. PCR Protocols, A Guide to Methods and Applications. Academic Press, San Diego, CA: 482 pp. [Google Scholar]
- Jones DB, Giles KL, Chen Y and Shufran KA, 2005. Estimation of Hymenopteran parasitism in cereal aphids using molecular markers. J. Econ. Entomol. 98: 217–221. [DOI] [PubMed] [Google Scholar]
- Kazmer DJ, Hopper KR, Coutinot DM and Heckel DG, 1995. Suitability of random amplified polymorphic DNA for genetic markers in the aphid parasitoid, Aphelinus asychis Walker. Biol. Control 5: 503–512. [Google Scholar]
- Knutson A, Boring EP III, Michels GJ Jr. and Gilstrap F, 1993. Biological Control of Insect Pests in Wheat. Texas Agric. Ext. Service Publ. B-5044, College Station, TX: 8 pp. [Google Scholar]
- Kwok S, Kellogg DE, McKinney N, Spasic D, Goda L, Levenson C and Sninsky JJ, 1990. Effect of primer-template mismatched on the polymerase chain reaction: human immunodeficiency virus type 1 model studies. Nuc. Acids Res. 18: 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matcham EJ and Hawkes C, 1985. Field assessment of the effects of deltamethrin on polyphagous predators in winter wheat. Pestic. Sci. 16: 317–320. [Google Scholar]
- Matejko I and Sullivan DJ, 1984. Interspecific tertiary parasitoidism between two aphid hyperparasitoids: Dendrocerus carpenteri and Alloxysta megourae (Hymenoptera: Megaspilidae and Cynipidae). J. Wash. Acad. Sci. 74: 31–38. [Google Scholar]
- Pike KS, Starý P, Miller T, Allison D, Boydston L, Graf G and Gillespie R, 1997. Small grain aphid parasitoids of Washington: distribution, relative abundance, and seasonal occurrence; and key to North American species. Environ. Entomol. 26: 1299–1311. [Google Scholar]
- Pike KS, Starý P, Miller T, Graf G, Allison D, Boydston L and Miller R, 2000. Aphid parasitoids (Hymenoptera: Braconidae: Aphidiinae) of Northwest USA. Proc. Entomol. Soc. Wash. 102: 688–740. [Google Scholar]
- Prinsloo G, Chen Y, Giles KL and Greenstone MH, 2002. Release and recovery in South Africa of the exotic aphid parasitoid Aphelinus hordei (Hymenoptera: Aphelinidae) verified by the polymerase chain reaction. BioControl 47: 127–136. [Google Scholar]
- Reed DK, Webster JA, Jones BG and Burd JD, 1991. Tritrophic relationships of Russian wheat aphid (Homoptera: Aphididae) and a hymenopterous parasitoid (Diaretiella rapae McIntosh), and resistant and susceptible small grains. Biol. Control 1: 35–41. [Google Scholar]
- Rider SD, Dobesh-Beckman SM and Wilde GE, 1998. Genetics of esterase mediated insecticide resistance in the aphid Schizaphis graminum. Heredity 81: 14–19. [Google Scholar]
- Royer TA, Giles KL, Kindler SD and Elliott NC, 2001. Developmental response of three geographic isolates of Lysiphlebus testaceipes (Hymenoptera: Aphidiidae) to temperature. Environ. Entomol. 30: 637–641. [Google Scholar]
- Scholz D and Höller C, 1992. Competition for hosts between two hyperparasitoids of aphids, Dendrocerus carpenteri and Dendrocerus laticeps (Hymenoptera: Megaspilidae): the benefits of interspecific host discrimination. J. Insect Behav. 3: 289–299. [Google Scholar]
- Starks KJ, Burton RL, Teetes GL and Wood EA Jr., 1976. Release of Parasitoids to Control Greenbugs on Sorghum. U.S. Dept. Agric., Agric. Res. Serv. ARS-S-91. 12 pp. [Google Scholar]
- Sullivan DJ and Völkl W, 1999. Hyperparasitism: multitrophic ecology and behavior. Annu. Rev. Entomol. 44: 291–315. [DOI] [PubMed] [Google Scholar]
- Sullivan DJ, 1988. Hyperparasites In: Helle W, Minks AK and Harrewijn P (eds), World Crop Pests – Aphids, their Biology, Natural Enemies and Control. Elsevier, New York: pp. 189–203. [Google Scholar]
- Teetes GL, Schaefer CA, Gipson JR, McIntyre RC and Latham EE, 1975. Greenbug resistance to organophosphorous insecticides on the Texas High Plains. J. Econ. Entomol. 68: 214–216. [Google Scholar]
- Walton MP, Powell W, Loxdale HD and Allen-Williams L, 1990. Electrophoresis as a tool for estimating levels of hymenopterous parasitism in field populations for the cereal aphid, Sitobion avenae. Entomol. Exp. Applic. 54: 271–279. [Google Scholar]
- Weathersbee AA, Shufran KA, Panchal T, Dang PM and Evans GA, 2004. Detection and differentiation of parasitoids (Hymenoptera: Aphidiidae and Aphelinidae) of the brown citrus aphid (Hemiptera: Aphididae): species-specific PCR amplification of 18S rDNA. Ann. Entomol. Soc. Am. 97: 286–292. [Google Scholar]
- Wright LC and James DG, 2001. Parasitoids of the hop aphid (Homoptera: Aphididae) on Prunus during the spring in Washington State. J. Agric. Urban Entomol. 18: 141–147. [Google Scholar]
- Zhang D-X and Hewitt GM, 1996. Nuclear integrations: challenges for mitochondrial DNA markers. Trends Ecol. Evol. 11: 247–251. [DOI] [PubMed] [Google Scholar]
- Zhu Y-C and Greenstone MH, 1999. Polymerase chain reaction techniques for distinguishing three species and two strains of Aphelinus (Hymenoptera: Aphelinidae) from Diuraphis noxia and Schizaphis graminum (Homoptera: Aphididae). Ann. Entomol. Soc. Am. 92: 71–79. [Google Scholar]
- Zhu Y-C, Burd JD, Elliott NC and Greenstone MH, 2000. Specific ribosomal DNA markers for early PCR detection of Aphelinus hordei (Hymenoptera: Aphelinidae) and Aphidius colemani (Hymenoptera: Aphidiidae) from Diuraphis noxia (Homoptera: Aphididae). Ann. Entomol. Soc. Am. 93: 486–491. [Google Scholar]