Corresponding Author

Key Words: COVID-19, myocardial injury, myocarditis
The past 6 months have been dominated by the highly contagious (R0 of ∼3) (1) severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) and the highly morbid and mortal coronavirus disease-2019 (COVID-19) pandemic that it has triggered. Early on, specific populations were identified to be at a greater risk for severe disease. Unsurprisingly, those with older age were among those at greater risk, though also prominent were individuals with cardiovascular disease (i.e., patients with coronary artery disease, heart failure, and atrial fibrillation) and even patients with cardiovascular risk factors (diabetes mellitus and hypertension). The pathophysiology associated with this increased risk may be linked to the role of the human angiotensin-converting enzyme 2 receptor as the portal of cell entry by SARS-CoV-2. However, whether the increased risk is because of the cardiovascular disease itself or medications used to treat it was initially unclear. Recent reports have not demonstrated an increased risk of severe COVID-19 infection associated with the use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (2,3), and presently it is not recommended to alter medical therapy for patients with cardiovascular disease prior to or during COVID-19.
Originally, COVID-19 was believed to be a predominantly respiratory disease; however, mounting evidence has identified that extrapulmonary manifestations, frequently cardiovascular manifestations, play a central role in disease progression and patient outcomes. The most basic evidence of myocardial involvement is troponin elevation, representing myocardial necrosis. Early reports from Wuhan, China, demonstrated a link between troponin elevation and increased risk for the need for mechanical ventilation or mortality (Table 1 ) (4, 5, 6). In this issue of the Journal, Lala et al. (7) report the prevalence, longitudinal change, and risk associated with troponin I elevation in 2,736 patients admitted to 5 hospitals in New York City. Patients were stratified into 3 groups based on troponin I level at presentation: normal (<0.03 ng/ml), low (0.03 to 0.09 ng/ml), and high (>0.09 ng/ml). Of note, troponin I was elevated in 36% of patients, which is higher than the previous reports from China. Cardiovascular disease (which included coronary artery disease, atrial fibrillation, and congestive heart failure) was more prevalent in patients with high levels of troponin I than in the other 2 patient groups. Even mild elevations of troponin were associated with an increased risk of mortality, with an adjusted hazard ratio of 1.75 (95% confidence interval: 1.37 to 2.24), while higher levels of troponin increased the risk of mortality further, with a hazard ratio of 3.03 (95% confidence interval: 2.42 to 3.80). In a subgroup of patients with multiple troponin measurements, mortality was greater in patients with a rising troponin following admission than in those in whom the troponin level declined following admission (7). The current report further supports the finding that myocardial injury represents a significant risk for mortality; however, it also reports for the first time that the severity of myocardial involvement as assessed by the magnitude of troponin I elevation further differentiates a patient’s risk for adverse outcomes. This large observational cohort provides the opportunity to develop a deeper understanding of the link between myocardial involvement and risk factors associated with severe COVID-19. As demonstrated here, atrial fibrillation, coronary artery disease, heart failure, diabetes mellitus, and hypertension not only were all significantly associated with elevated troponin I overall, but also increased in prevalence as the magnitude of myocardial injury increased.
Table 1.
Published Studies Worldwide Demonstrating Association Between Myocardial Injury Diagnosed by Troponin Elevation and the Association With COVID-19–Associated Mortality
| Location | N | Patient Acuity | Assay Used | HR (95% CI) for Death | Prevalence in Nonsurvivors vs. Survivors | Ref. # |
|---|---|---|---|---|---|---|
| Wuhan, China | 671 | Severe | hs-cTnI | 4.56 (1.28–16.28) | 75.8% vs. 9.7% | (4) |
| Wuhan, China | 416 | Hospitalized | hs-cTnI | 4.26 (1.92–9.49) | 51.2% vs. 4.5% | (5) |
| Wuhan, China | 191 | Hospitalized | hs-cTnI | 80.1 (10.3–620.36) | 46% vs. 1% | (6) |
| Seattle, United States | 24 (13 with measured troponin) | Severe | Troponin (not otherwise specified) | 50% (n = 1 of 2) vs. 45% (n = 5 of 11) | (9) | |
| Northern Italy | 53 | Hospitalized with pre-existing CVD | hs-cTnT | 100% vs. 74% | (10) | |
| New York City, United States | 2,736 | Hospitalized | Troponin I | Low (0.03–0.09 ng/ml): 1.75 (1.37–2.24) High (>0.09 ng/ml): 3.03 (2.42–3.80) |
60% (>0.09 ng/ml) vs. 35% (0.03–0.09 ng/ml) vs. 15% (<0.03 ng/ml) (estimated from Figure 1 of Lala et al.) | (7) |
CI = confidence interval; COVID-19 = coronavirus disease-2019; CVD = cardiovascular disease; HR = hazard ratio; hs-cTnI = high-sensitivity cardiac troponin I; hs-cTnT = high-sensitivity cardiac troponin T.
Recent reports of a multisystem inflammatory syndrome with features of Kawasaki disease in children have further linked cardiovascular injury to COVID-19 (8). It is possible that the inflammatory syndrome associated with COVID-19 in general and antibody-mediated disease in specific may cause vasculitis.
Although cardiovascular disease clearly represents a risk factor for the development and severity of COVID-19, cardiovascular disease may also be caused and exacerbated by COVID-19:
-
1.
Increased risk for myocardial ischemia: The sympathetic activation, leading to increased myocardial oxygen demand in conjunction with hypoxemia with reduced myocardial oxygen supply, may lead to imbalance in the myocardial oxygen demand-to-supply ratio. In addition, hypercoagulability, systemic proinflammatory state (cytokine storm), vasculitis, and direct vascular infection may increase the risk for plaque rapture and infarction.
-
2.
Increased risk for development of heart failure with a reduced ejection fraction: Similar to the increased risk for myocardial ischemia, the cytokine storm may lead to myocardial depression and direct myocardial infection (myocarditis), further increasing the risk for myocardial necrosis and heart failure.
-
3.
Increased risk for arrhythmias: Myocardial ischemia, myocarditis, increased sympathetic tone, inflammation, and electrolyte imbalance all lead to increase risks for both atrial and ventricular arrhythmia.
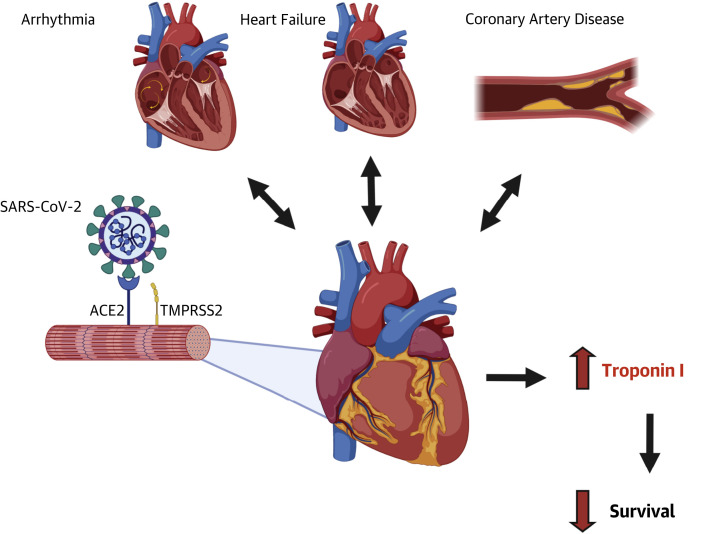
The main question that arises is whether COVID-19 is a disease that originates as a cardiovascular disease or whether cardiovascular involvement is a downstream consequence of severe COVID-19 (Figure 1 ). As previously discussed, it remains uncertain which comes first, and it may be more reasonable to suspect that both types presentation are possible. Some patients may experience cardiovascular symptoms as their initial manifestations; however, others may experience cardiovascular involvement only as the disease progresses. Irrespectively, involvement of the cardiovascular system represents a more severe form of COVID-19, and as presented here by Lala et al. (7), significant numbers of hospitalized patients experience myocardial injury, and a greater magnitude of injury portends a worse prognosis for the patient.
Figure 1.
Interplay of Cardiovascular Disease and SARS-CoV-2
The interplay of pre-existing cardiovascular disease (arrhythmia, heart failure, coronary artery disease) and severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) (pictured with spike protein binding to angiotensin-converting enzyme 2 receptor) on the heart. ACE2 = angiotensin-converting enzyme 2; TMPRSS2 = transmembrane protease, serine 2.
COVID-19 has forced us to understand the disease pathophysiology and identify associated risk factors in a short period. As our knowledge evolves and we attempt to provide the best care for our patients, risk stratification is crucial. Lala et al. have furthered our understanding of COVID-19 and demonstrated that troponin may serve as useful tool to achieve this goal.
Acknowledgment
The authors acknowledge BioRender for providing templates and the platform that were used for creating the figure.
Footnotes
Dr. Clerkin is supported by the National Heart, Lung, and Blood Institute (Grant K23HL148528). The authors have reported that they have no relationships relevant to the contents of this paper to disclose. Patrick O’Gara, MD, served as Guest Associate Editor for this paper. P.K. Shah, MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACCauthor instructions page.
References
- 1.Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27:taaa021. doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fosbøl E.L., Butt J.H., Østergaard L. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA. 2020 Jun 19 doi: 10.1001/jama.2020.11301. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds H.R., Adhikari S., Pulgarin C. Renin–angiotensin–aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi S., Qin M., Cai Y. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41:2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 Mar 25 doi: 10.1001/jamacardio.2020.0950. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lala A., Johnson K.W., Januzzi J.L. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung E.W., Zachariah P., Gorelik M. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020 Jun 8 doi: 10.1001/jama.2020.10374. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inciardi R.M., Adamo M., Lupi L. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41:1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]