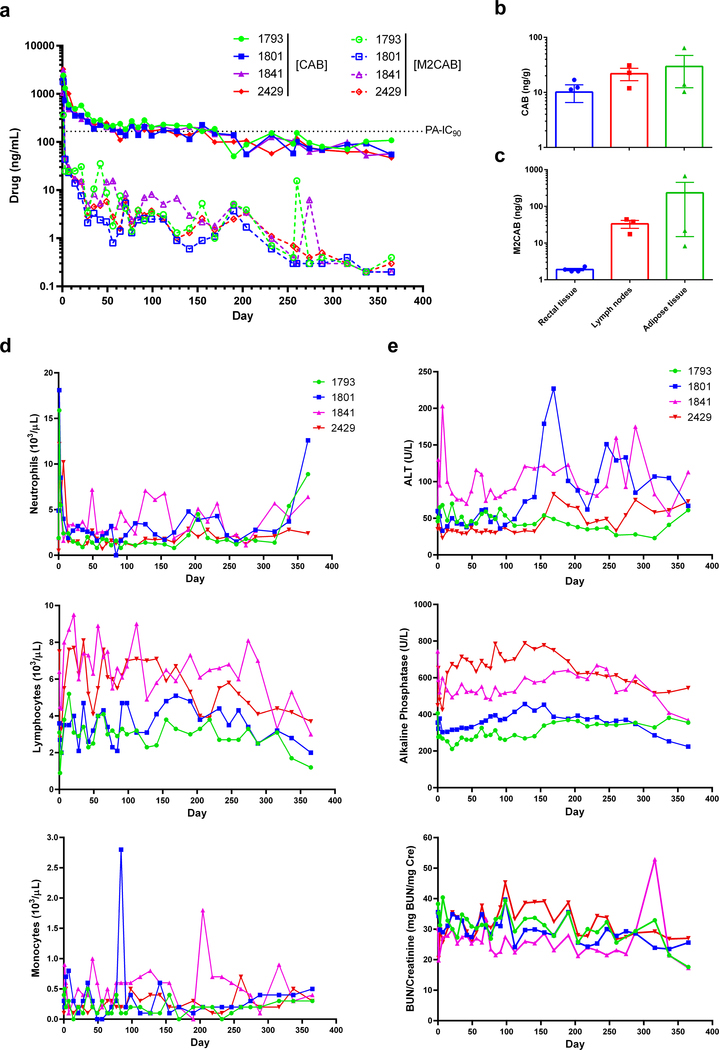
Figure 6. PK, BD, and toxicological assessments in rhesus macaques.
Four rhesus macaques were administered a 45 mg/kg CAB-equivalent dose of NM2CAB by a single IM injection. (a) Plasma samples were collected, and CAB and M2CAB levels were determined up to day 365. (b and c) Rectal, lymph node, and adipose tissue biopsies were collected at day 204 following drug administration and assayed for (b) CAB and (c) M2CAB concentrations. (d and e) Systemic adverse reactions were evaluated by measuring (d) hematologic and (e) metabolic profiles. Plasma drug and prodrug concentrations, and hematologic and metabolic profile parameters are shown for individual animals. Tissue drug concentrations are expressed as mean ± SEM; N = 4 animals.