FIGURE 1.
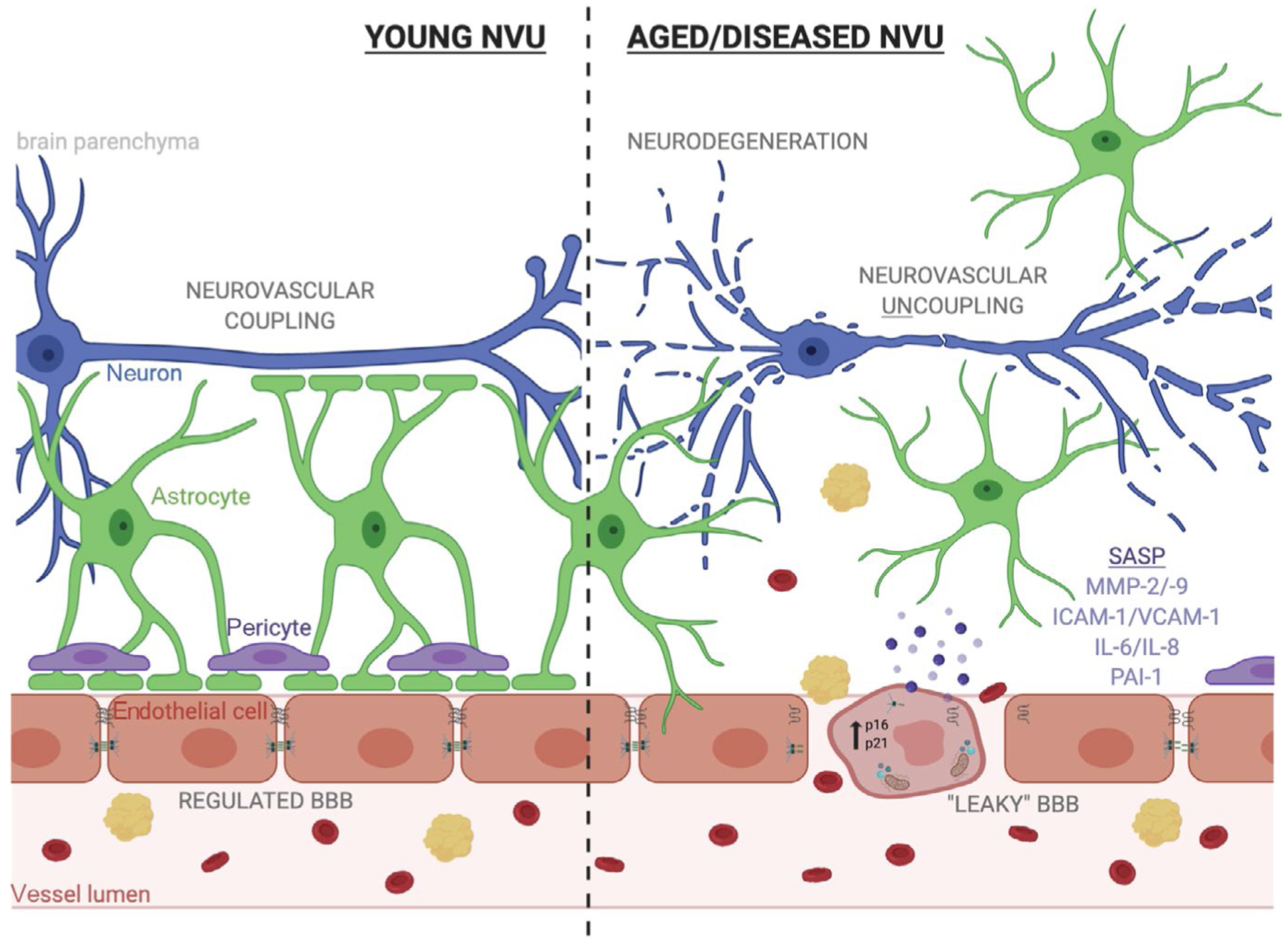
Endothelial cell senescence could drive blood-brain barrier (BBB) disruption, neurovascular uncoupling and neurodegeneration in ageing and disease. The neurovascular unit (NVU) is composed of endothelial cells, astrocytes, pericytes, and neurons which function cooperatively to regulate molecular transport from the periphery to the brain in order to maintain neuronal homeostasis and respond to changes in neuronal energy demands. Aging can induce changes in endothelial cells that disrupt the NVU’s role in the BBB and neurovascular coupling. These changes to endothelial cells are reminiscent of cellular senescence and include upregulation of cyclin-dependent kinase inhibitors, acquisition of a pro-inflammatory and degradative senescence-associated secretory phenotype (SASP), increased oxidative stress, and deregulated tight junctions. In this way, endothelial cell senescence along with age-related loss of pericytic coverage of vessels can lead to increased extravasation of red blood cells and other neurotoxic proteins. This BBB disruption causes an imbalance in the local microenvironment and can drive neuronal dysfunction. Additionally, age-related increases in astrocytic reactivity result in loss of astrocytic endfoot coverage and break the communication between neurons and the endothelium, termed “neurovascular uncoupling”, and also drives neurodegeneration
