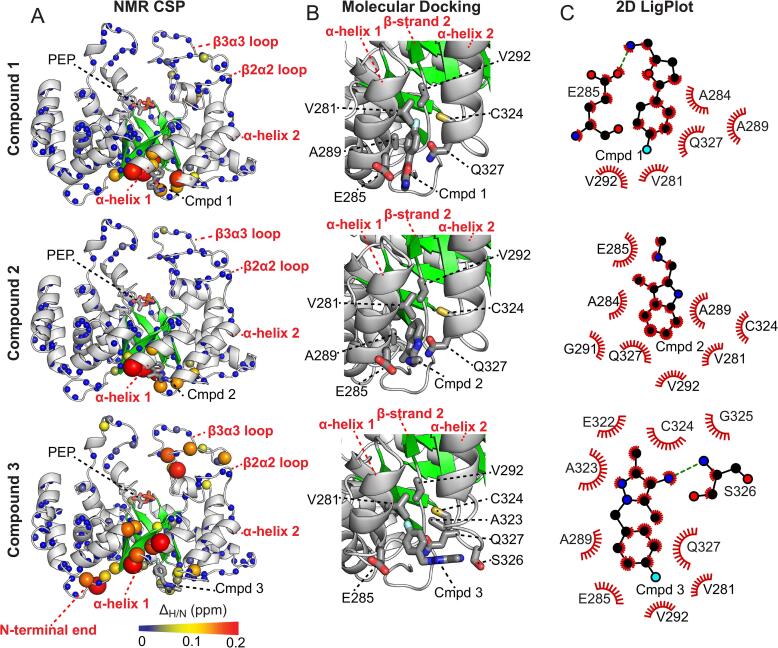
Fig. 4.
Structures of the EIC-inhibitor complexes. (a) Weighted combined chemical shift perturbations (ΔH/N) induced by 8 mM of compound 1 (top), 2 (center) and 3 (bottom) on the 1H-15N HSQC spectrum of EIC. ΔH/N values are displayed on the structure of the EIC-PEP complex as spheres with the relationship between size and color of each sphere and chemical shift perturbation depicted by the color bar. The PEP molecule is shown as solid sticks. Structure and localization of compounds 1, 2, and 3 resulting from molecular docking calculation is also displayed as solid sticks. (b) Close-up view of the inhibitor binding site. Inhibitors and EIC side-chains involved in complex formation are shown as solid sticks. (c) 2D ligand–protein interaction diagrams of the EIC-inhibitor complexes highlighting hydrophobic contacts (red) and hydrogen-bonding (dashed green line) interactions. Plots were generated using the program LigPlot (Wallace et al., 1995). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)