Abstract
Triple‐negative breast cancer (TNBC) is an aggressive subtype of breast cancer compared with luminal or epidermal growth factor receptor 2 subtypes, thus effective therapeutic options for TNBC are yet to be developed. Nowadays, oncogenic long noncoding RNAs (lncRNAs) are applied to cancer management as a new class of therapeutic targets. We previously showed that thymopoietin antisense transcript 1 (TMPO‐AS1) is a proliferation‐associated lncRNA that contributes to hormone‐dependent breast cancer progression by stabilizing estrogen receptor‐α mRNA. We here showed that TMPO‐AS1 is abundantly expressed in basal‐like breast cancer subtype based on the transcriptomic data in The Cancer Genome Atlas as well as in TNBC cell lines and patient‐derived cells. Small interfering RNA‐based loss‐of‐function analyses showed that TMPO‐AS1 knockdown substantially represses the proliferation and migration of TNBC cells. Expression microarray analysis showed that TMPO‐AS1 alters gene signatures related to transforming growth factor‐β signaling in addition to proliferative E2F signaling pathways. TMPO‐AS1‐targeted siRNA treatment through engineered drug delivery systems using cancer‐targeted polyion complex micelle or nanoball technology significantly impaired the in vivo growth of primary and metastatic TNBC xenograft tumors. Our findings suggest that TMPO‐AS1 plays a key role in TNBC pathophysiology and could be a potential therapeutic target for TNBC.
Keywords: breast cancer, DDS, lncRNA, TMPO‐AS1, TNBC
TMPO‐AS1, a long noncoding RNA, regulates proliferation and migration of triple‐negative breast cancer (TNBC) cells with modulating transforming growth factor‐β and E2F signaling pathways. An engineered drug delivery system using cancer‐targeted polyion complex micelle or nanoball technology is useful for TMPO‐AS1 siRNA in xenograft therapeutic models of TNBC. TMPO‐AS1 plays a key role in TNBC pathophysiology and could be a potential therapeutic target for TNBC.
Abbreviations
- ATCC
American type culture collection
- CHEK1
checkpoint kinase 1
- cRGD
cyclic RGDfK peptide
- DDS
drug delivery system
- DGL
dendrigraft poly‐L‐lysine
- DMEM
Dulbecco's modified Eagle's medium
- ER
estrogen receptor
- ESR1
estrogen receptor 1
- GAPDH
glyceraldehyde‐3‐phosphate dehydrogenase
- GEO
Gene Expression Omnibus
- GO
Gene Ontology
- GSEA
Gene set enrichment analysis
- HER2
human epidermal growth factor receptor 2
- IM
2‐iminothiolane
- LAP2
lamina‐associated polypeptide 2
- lncRNA
long noncoding RNA
- MAD2L1
mitotic arrest deficient 2 like 1
- MCM6
minichromosome maintenance protein complex component 6
- MKi‐67
marker of proliferation Ki‐67
- MPA
1‐(3‐mercaptopropyl)amidine, PCNA, proliferating cell nuclear antigen
- PEG
poly(ethylene glycol), PI, propidium iodide
- PIC/m
polyion complex micelle
- PLL
poly(L‐lysine)
- PR
progesterone receptor
- rRNA
18S ribosomal RNA
- STR
short tandem repeat
- TCGA
The Cancer Genome Atlas
- TGFBR1
transforming growth factor beta receptor 1
- TGFBR2
transforming growth factor beta receptor 2
- TGF‐β
transforming growth factor beta
- TMPO‐AS1
thymopoietin antisense transcript 1
- TNBC
triple‐negative breast cancer
- γ‐PGA
γ‐polyglutamic acid
1. INTRODUCTION
Breast cancer is the most common type of cancer in women and the number of breast cancer patients is on the rise worldwide. 1 Breast cancer is categorized as subtypes by expression markers such as hormone receptors, including ER and progesterone receptor, and HER2. 2 These expression markers are essential for the development and progression of each type of cancer and utilized for clinical therapies. 3 For example, the most predominant type of breast cancer, ER‐positive breast cancer, is treated with antiestrogen reagents such as tamoxifen as a fundamental therapeutic option. 4 In addition, HER2 Ab is a useful treatment method for HER2‐positive breast cancer patients. 5 However, a breast cancer subtype that does not express these markers, denoted TNBC, accounts for 10%‐24% of all breast cancer cases. 6 Unfortunately, the only fundamental option for the treatment of TNBC is standard chemotherapy, as specific molecular targeted therapy is underdeveloped. Furthermore, TNBC is more aggressive and metastatic compared with other types of breast cancer 7 ; therefore, the characterization of new factors involved in the development and progression of TNBC is greatly anticipated.
A number of lncRNAs have been reported to be associated with various biological phenomena, immune reactions, neuronal diseases, and cancer development. 8 , 9 , 10 Long noncoding RNA, by definition, is longer than 200 nucleotides and does not code for any structured protein. 11 Long noncoding RNAs modulate signaling pathways by binding to their target partners, which include protein, DNA, and RNA molecules. 12 , 13 Several lncRNAs have been reported to be involved in TNBC cell proliferation and metastasis through elaborate mechanisms. 14 , 15 , 16 In our previous study, we characterized TMPO‐AS1 as an lncRNA strongly associated with cell proliferation markers, including MKi‐67 and PCNA. 17 TMPO‐AS1 was originally identified as a downstream lncRNA of E2F signaling. 18 We showed that TMPO‐AS1 promotes ER‐positive breast cancer cell proliferation and antiestrogen therapy resistance through stabilizing ESR1 RNA. However, the role of TMPO‐AS1 in TNBC has not been addressed.
We showed that the intratumoral injection of siTMPO‐AS1 efficiently impairs in vivo growth of s.c. tumors derived from ER‐positive breast cancer cells in a mouse xenograft model. RNA interference‐mediated medicine is applied to cancer management as an efficient molecular targeting therapy as nucleic acid drugs can be easily designed by targeting specific sequences for individual genes and RNAs. 19 In the case of siRNA, however, it remains to be solved in terms of its instability and difficulty in delivery to specific target cells. To overcome these drawbacks, DDS has been developed. 20 The purpose of DDS includes enhancing the stability of siRNA and the specificity of siRNA delivery, leading to maximized therapeutic impact of siRNA with reduced side‐effects. 21 , 22
In the present study, we examine the tumorigenic function of TMPO‐AS1 in TNBC using patient‐derived cells as well as known TNBC cell lines. As siRNAs against TMPO‐AS1 could efficiently repress the proliferation and migration of TNBC cells, we evaluated the therapeutic potential of these siRNAs in mouse xenograft models based on recently developed nanoparticulate DDS.
2. MATERIALS AND METHODS
2.1. Cell culture and reagents
Human TNBC breast cancer cell lines MDA‐MB‐231 and MDA‐MB‐468 (ATCC) were authenticated by short tandem repeat analysis at BEX and cultured in DMEM containing 10% FBS, 50 units/mL penicillin, and 50 μg/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2. For TMPO‐AS1 overexpression, pcDNA3‐empty or pcDNA3‐TMPO‐AS1 vectors were transfected in MDA‐MB‐231 cells with Lipofectamine 2000 (Invitrogen) and used for further experiments. MDA‐MB‐231 cells were transfected with pGL3‐Promoter vector (Promega) and selected with G418 to establish luciferase‐expressing MDA‐MB‐231 cells. The TNBC‐PDC were established from tumor tissue of a TNBC patient as described previously 23 , 24 and cultured in DMEM containing 10% FBS, 50 units/mL penicillin, and 50 μg/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2.
2.2. Bioinformatics
TMPO‐AS1 expression data in TCGA breast cancer dataset 25 were obtained and analyzed by TANRIC software (https://www.tanric.org). 26 TMPO‐AS1‐coexpressed genes in TCGA breast cancer dataset were determined at a threshold of greater than 0.4 by Spearman’s correlation using cBioportal software (https://www.cbioportal.org) 27 , 28 The enrichment of pathways among the retrieved TMPO‐AS1‐coexpressed genes was evaluated by GO analysis with biological process GO terms (https://david.ncifcrf.gov/) 29
2.3. Quantitative real‐time PCR
Total RNA was extracted from cells using ISOGEN reagent (Nippon Gene), followed by cDNA synthesis using SuperScript III reverse transcriptase (Invitrogen) with random primers. Quantitative RT‐PCR was carried out on a StepOnePlus Real‐Time PCR System (Thermo Fisher Scientific) using KAPA SYBR FAST qPCR Kit (KAPA Biosystems) and sets of gene‐specific primers. RNA expression levels were analyzed by the ΔΔC t method and normalized to the values of GAPDH or 18S ribosomal RNA. The sequences of primers are listed in Table S1.
2.4. Small interfering RNA transfection
Small interfering RNAs against TMPO‐AS1 were designed using siDirect and purchased from RNAi Inc. A negative control siRNA (siControl) with no homology to known gene targets in mammalian cells was purchased from RNAi Inc. MDA‐MB‐231 and TNBC‐PDC cells were seeded at 200 000‐300 000 cells per well in 6‐well plates and simultaneously transfected with siRNA at a final concentration of 10 nmol/L using Lipofectamine RNAiMAX (Invitrogen). Cells were harvested 48‐72 hours after transfection and used for qRT‐PCR, cell cycle analysis, and annexin V and PI staining. The sequences of siRNA are as follows (forward and reverse, respectively): siTMPO‐AS1 #1 (5′‐gaagacuagugaccuauaauu‐3′ and 5′‐uuauaggucacuagucuuccu‐3′) and siTMPO‐AS1 #2 (5′‐gagccgaacuacgaaccaacu‐3′ and 5′‐uugguucguaguucggcucug‐3′).
2.5. DNA assay
Cells were seeded and treated with siRNAs at the indicated densities (3000 cells per well for MDA‐MB‐231 and 1500 cells per well for TNBC‐PDC) in 96‐well plates. Cells were harvested at 1, 3, and 5 days, lysed with freeze‐and‐thaw, and buffered with 5 mmol/L Tris‐HCl, pH 7.5, containing 1 mol/L NaCl and 0.5 mmol/L EDTA. Extracted DNA was stained with Hoechst 33 258 pentahydrate (Thermo Fisher Scientific) at a final concentration of 5 μg/mL. DNA content in each well was measured on ARVO5 (Perkin Elmer) at 355 nm for 0.1 seconds.
2.6. Cell cycle analysis
The siRNA‐treated cells were harvested and fixed with 70% ethanol for 30 minutes. Fixed cells were treated with RNase A and stained with 5 μg/mL PI. DNA content was measured using FACSCalibur (Becton Dickinson). Data were analyzed by CellQuest software (Becton Dickinson) to determine the percentage of cells in the G1, S, and G2/M phases.
2.7. Annexin V and PI staining
After transfection with siRNAs, cells were harvested, and apoptotic cells were stained using the FITC annexin V apoptosis detection kit (Becton Dickinson) following the manufacturer’s instructions. Percentages of apoptotic cells were analyzed on FACSCalibur (Becton Dickinson).
2.8. Microarray and pathway analysis
For microarray analysis, human Clariom D array (Thermo Fisher Scientific) was used following the manufacturer’s instructions. Data were analyzed using Affymetrix Microarray Suite software. All microarray data are available in the Gene Expression Omnibus (GEO) database with the accession number GSE141925. Pathway analyses were undertaken using GSEA.
2.9. Migration assay
MDA‐MB‐231 and TNBC‐PDC cells were seeded at 100 000 cells per well in a 24‐well plate and simultaneously transfected with siRNAs at a final concentration of 10 nmol/L for 48 hours. Cells were reseeded onto a polycarbonate membrane insert in a Transwell apparatus (Costar). Twenty‐four hours after incubation, migrated cells were fixed with methanol and stained with Giemsa solution. Stained cells were counted under a microscope in 5 independent fields (×200).
2.10. Preparation of siRNA‐loaded PIC/m
A cancer‐targeted PIC/m was prepared from siRNA and cRGD‐installed PEG‐block‐PLL functionalized with MPA and IM (cRGD‐PEG‐PLL(MPA/IM)), as described previously. 30 , 31 cRGD‐PEG‐PLL(MPA/IM) (molecular weight of PEG, 12 000; degree of polymerization of PLL, 38) was dissolved in 10 mmol/L HEPES buffer (pH 7.3), mixed with DTT solution, and then incubated for 0.5 hours. Thereafter, the polymer solution was mixed with siRNA dissolved in the same buffer at a molar ratio of cRGD‐PEG‐PLL to siRNA of approximately 8. The mixture was dialyzed against 10 mmol/L HEPES buffer (pH 7.3) containing 0.5% DMSO for 48 hours and 10 mmol/L HEPES buffer for 48 hours. The obtained PIC/m was characterized to have a hydrodynamic diameter of 35 nm with a polydispersity index of 0.08 by dynamic light scattering.
2.11. Subcutaneous in vivo tumor formation and siRNA treatment
All animal experiments were approved by the Animal Care and Use Committee of Saitama Medical University, and carried out in accordance with the Guidelines and Regulations for the Care and Use of Experimental Animals of Saitama Medical University. Female BALB/cAJcl‐nu/nu mice were purchased from CLEA Japan. MDA‐MB‐231 cells (2 million cells per mouse) were mixed with an equal volume of Matrigel matrix (Corning) and injected s.c. into the flank of 10‐week‐old female mice. When the average tumor volume exceeded 200 mm3, mice were randomly assigned to receive either siControl (n = 5) or siTMPO‐AS1 #2 (n = 6). The siRNA samples (25 μg) encapsulated in PIC/m were i.v. injected into the tail vein twice a week for 4 weeks. Three dimensions of tumor were measured once a week and tumor volumes were estimated according to the formula: 0.5 × ([smallest diameter]2 × [longest diameter]). At the end‐point of the experiment, tumors were dissected from the mice.
2.12. Lung‐selective nanoball‐siRNA preparation
Lung‐selective nanoball was prepared as follows. 32 The siRNA solution was mixed with DGL solution and γ‐PGA solution by thorough pipetting to construct lung‐selective nanoball‐siRNA, before being left for 30 minutes at room temperature. The obtained nanoball was characterized to have a hydrodynamic diameter of approximately 100 nm with a polydispersity index of 0.2 by dynamic light scattering. Also, the nanoball had anionic surface charge (approximately −20 mV).
2.13. Lung metastatic tumor model and siRNA treatment
Female NOD/SCID mice were purchased from CLEA Japan. MDA‐MB‐231‐luc cells stably expressing luciferase (2 million cells per mouse) were i.v. injected into 10‐week‐old female mice through tail veins. Fifty micrograms of siControl (n = 8) or siTMPO‐AS #2 (n = 9) were mixed with lung‐selective nanoball 30 and i.v. injected into tail veins twice a week for 2 weeks. To count fluorescence intensities, mice were i.p. injected with D‐luciferin (3 mg per mouse), anesthetized for 5 minutes with isoflurane, and imaged for 2 minutes under anesthesia using the in vivo imaging system (Photon Imager; Biospace Lab). In vivo imaging data were analyzed with the Photo Vision software (Bio Space) for the quantification of data.
2.14. Statistical analysis
Comparisons between the 2 groups were analyzed by Student’s t test. Two‐way ANOVA was undertaken to analyze differences among siRNA treatment groups.
3. RESULTS
3.1. Proliferation‐associated lncRNA, TMPO‐AS1, is abundantly expressed in TNBC cells
We previously reported that, among lncRNAs, TMPO‐AS1 shows the most significant coexpression pattern with proliferation markers (MKI67 and PCNA) in the breast cancer TCGA dataset. 17 We showed that TMPO‐AS1 is important for the proliferation of ER‐positive breast cancer cells through the upregulation of ESR1 mRNA. Because the breast cancer TCGA dataset also includes TNBC samples, we had a notion that TMPO‐AS1 also plays an important role in TNBC cell proliferation. At first, we assessed TMPO‐AS1 expression levels in TNBC cells compared with those of estrogen‐treated and ‐untreated MCF‐7 cells, as TMPO‐AS1 was found as an estrogen‐inducible lncRNA (Figure 1A). TNBC cell lines such as MDA‐MB‐231 or MDA‐MB‐468 more abundantly express TMPO‐AS1 than estrogen‐untreated MCF‐7 cells (Figure 1A). In addition, TNBC‐patient‐derived cells (TNBC‐PDC), which were originally established from TNBC tumor cells, 23 , 24 also substantially expressed TMPO‐AS1 (Figure 1A). TMPO‐AS1 expression analysis in the TCGA dataset showed that TMPO‐AS1 is highly expressed in basal‐type as well as luminal B subtype breast cancer tissues as compared with normal‐like tissues (Figure 1B). We also showed that TMPO‐AS1‐coexpressed genes (Table S2) in the TCGA dataset were strongly associated with cell proliferation‐related pathways (Figure 1C).
FIGURE 1.
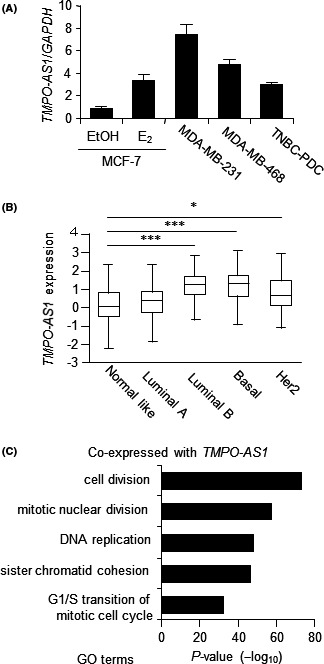
Proliferation‐associated long noncoding RNA, TMPO‐AS1, is abundantly expressed in triple‐negative breast cancer (TNBC) cells. A, TMPO‐AS1 levels in estrogen (E2)‐treated or ‐untreated MCF‐7 cells and TNBC cells were analyzed by quantitative real‐time PCR. Relative RNA levels were determined by the normalization to GAPDH levels based on the ΔΔC t method and each value is presented as mean fold change ± SD vs MCF‐7 cells with vehicle (EtOH) treatment. B, TMPO‐AS1 expression in The Cancer Genome Atlas breast cancer samples was analyzed by TANRIC software. C, TMPO‐AS1‐coexpressed genes were analyzed by gene ontology (GO) pathway analysis. Data show top 5 TMPO‐AS1‐coexpressed genes and their associated pathways
3.2. TMPO‐AS1 regulates TNBC cell proliferation
As TMPO‐AS1 expression is also elevated in basal subtype breast cancer, we postulated that TMPO‐AS1 is involved in the proliferation of TNBC cells. To test this hypothesis, we introduced siTMPO‐AS1 into TNBC cell line MDA‐MB‐231 (Figure 2A) and TNBC‐PDC (Figure 2B). The siRNA‐mediated knockdown of TMPO‐AS1 significantly suppressed the proliferation of both MDA‐MB‐231 (Figure 2C) and TNBC‐PDC (Figure 2D) cells. In addition, TMPO‐AS1 knockdown suppressed cell cycle progression (Figure 2E) and induced apoptotic cell death (Figure 2F). In contrast, TMPO‐AS1 overexpression (Figure S1A) promoted cell proliferation in MDA‐MB‐231 cells (Figure S1B).
FIGURE 2.
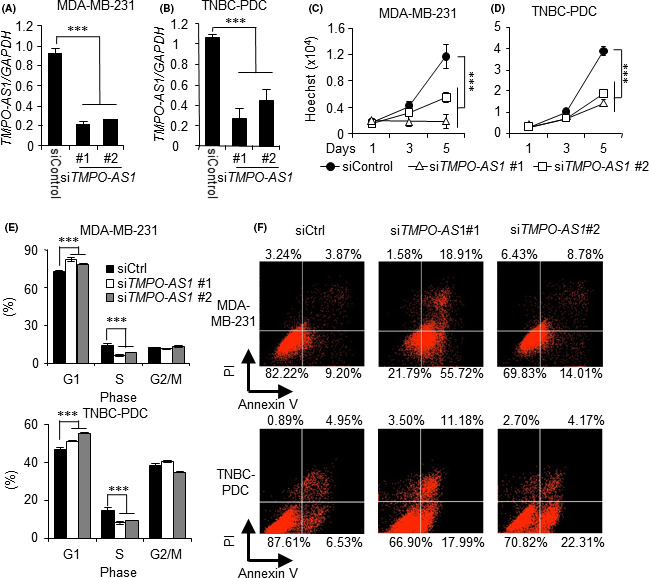
TMPO‐AS1 knockdown suppresses triple‐negative breast cancer (TNBC) cell proliferation. A, B, Knockdown efficiency of TMPO‐AS1 siRNAs in MDA‐MB‐231 (A) and TNBC‐patient‐derived (PDC) (B) cells analyzed by quantitative real‐time PCR. Relative RNA levels are presented as mean fold change ± SD vs negative control siRNA (siCtrl) in each cell type (n = 3). C, D, Viability of MDA‐MB‐231 (C) and TNBC‐PDC (D) cells on indicated days after siRNA treatment analyzed by DNA assay. Values are presented as mean ± SD vs siControl in each cell type (n = 5). E, Cell cycle profiles with propidium iodide (PI) of MDA‐MB‐231 (upper panel) and TNBC‐PDC (lower panel) cells treated with indicated siRNAs analyzed by flow cytometry. Percentages of cell populations in G1, S, and G2/M phases are shown (n = 3). F, Percentages of annexin V‐positive populations in MDA‐MB‐231 cells (upper panel) and TNBC‐PDC (lower panel) cells treated with indicated siRNAs analyzed by flow cytometry. *P < .05; **P < .01; ***P < .001
3.3. TMPO‐AS1 modulates E2F‐ and TGF‐β‐associated gene expression in TNBC cells
In the next step, we carried out microarray analysis in the TMPO‐AS1‐silenced MDA‐MB‐231 cells to understand the transcriptomic effect of TMPO‐AS1 on gene expression in TNBC cells. The GSEA‐mediated pathway analysis showed that TMPO‐AS1 knockdown altered cell proliferation‐associated gene expression such as “E2F targets” and “G2M checkpoint” (Figure 3A,B). In addition, TGF‐β signaling is strongly associated with TMPO‐AS1 (Figure 3A,B). We validated several gene expression profiles in the “E2F target” pathway; MCM6, TMPO, MAD2L1, and CHEK1 were silenced by siTMPO‐AS1 (Figure 3C). Furthermore, overexpression of TMPO‐AS1 increased these “E2F target” pathway‐related genes (Figure S2). We also showed that TGFBR1 and TGFBR2 levels were suppressed by TMPO‐AS1 knockdown (Figure 3D).
FIGURE 3.
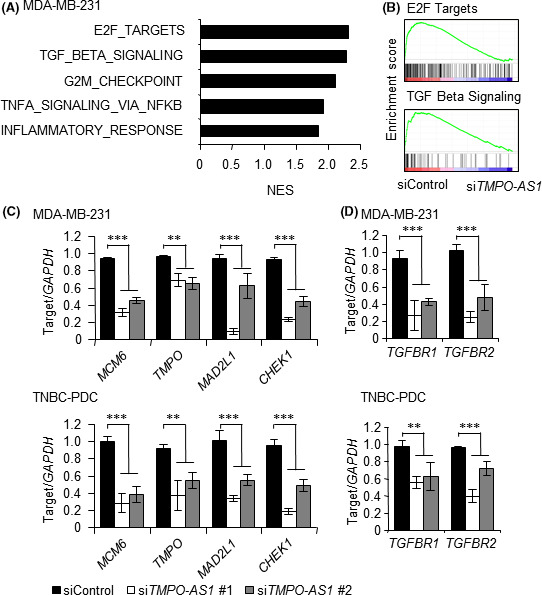
TMPO‐AS1 modulates E2F‐ and transforming growth factor‐β (TGF‐β)‐associated gene expression in triple‐negative breast cancer (TNBC) cells. A, Top 5 pathways enriched in genes downregulated by siTMPO‐AS1 vs negative control siRNA (siControl) determined by gene set enrichment analysis (GSEA) in MDA‐MB‐231 cells. B, GSEA enrichment plots for dominant pathways in MDA‐MB‐231 cells treated with siTMPO‐AS1, including “E2F targets” and “TGF‐β signaling”. C, Effects of TMPO‐AS1 knockdown on MCM6, TMPO, MAD2L1, and CHEK1 levels in MDA‐MB‐231 (upper panel) and TNBC‐patient‐derived (PDC) (lower panel) cells. Relative RNA levels are presented as mean fold change ± SD vs siControl (n = 3). D, Effects of TMPO‐AS1 knockdown on TGFBR1 and TGFBR2 levels in MDA‐MB‐231 (upper panel) and TNBC‐PDC (lower panel) cells. Relative RNA levels are presented as mean fold change ± SD vs siControl (n = 3). *P < .05; **P < .01; ***P < .001. NES, normalized enrichment score.
3.4. TMPO‐AS1 knockdown suppresses TNBC cell migration
Pathway analysis based on microarray results showed that TMPO‐AS1 is associated with the TGF‐β pathway as well as the cell proliferation pathway (Figure 3A,B). As the TGF‐β pathway is known to be associated with the metastasis of broad cancer types including breast cancer, 33 , 34 we assume that TMPO‐AS1 is also linked to a metastatic phenotype of TNBC. We showed that TMPO‐AS1 knockdown significantly suppressed the migration of MDA‐MB‐231 (Figure 4A) and TNBC‐PDC cells (Figure 4B). In contrast, TMPO‐AS1 overexpression increased the expression of TGFBR1 and TGFBR2 (Figure S3A,B), and promoted MDA‐MB‐231 cell migration (Figure S3C,D).
FIGURE 4.
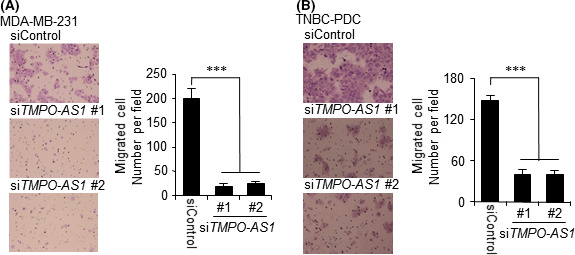
TMPO‐AS1 knockdown suppresses triple‐negative breast cancer (TNBC) cell migration. A, B, siTMPO‐AS1‐treated cells were seeded onto the Transwell membrane. Migrating cells were stained with Giemsa and counted under a microscope in 5 independent fields. Representative photograph of migrating MDA‐MB‐231 (A, left panel) and TNBC‐patient‐derived (PDC) (B, left panel) cells is shown. Summarized data of MDA‐MB‐231 (A, right panel) and TNBC‐PDC (B, right panel) cells are presented as mean ± SD (n = 5). *P < .05; **P < .01; ***P < .001. siControl, negative control siRNA
3.5. Polyion complex micelle‐TMPO‐AS1 siRNA treatment suppresses s.c. TNBC tumor growth
We next determined whether siTMPO‐AS1 could suppress in vivo growth of TNBC tumors. We previously showed that the intratumoral injection of siTMPO‐AS1 #2 with a conventional lipofection reagent could significantly suppress in vivo tumor growth derived from ER‐positive breast cancer cells. In this study, we undertook i.v. siRNA administration to nude mice with s.c. xenograft tumors based on cancer‐targeted DDS using cRGD peptide‐conjugated PIC/m. 30 , 31 , 35 When the volume of MDA‐MB‐231‐derived tumors in the flank of mice reached more than 200 mm3, i.v. injection of PIC/m‐mediated siTMPO‐AS1 (PIC/m‐siTMPO‐AS1) or negative control siRNA (PIC/m‐siControl) into the tail veins was carried out twice a week for 4 weeks. Tumor growth was significantly impaired by 8 injections of PIC/m‐siTMPO‐AS1 compared to the same number of injections of PIC/m‐siControl (Figure 5A,B). To confirm the TMPO‐AS1 knockdown effect in each tumor, we dissected tumors from each mouse and quantified the expression levels of TMPO‐AS1 and its downstream target genes. TMPO‐AS1 and its downstream target gene expression levels were decreased in PIC/m‐siTMPO‐AS1‐treated tumors compared with PIC/m‐siControl‐treated tumors (Figure 5C).
FIGURE 5.
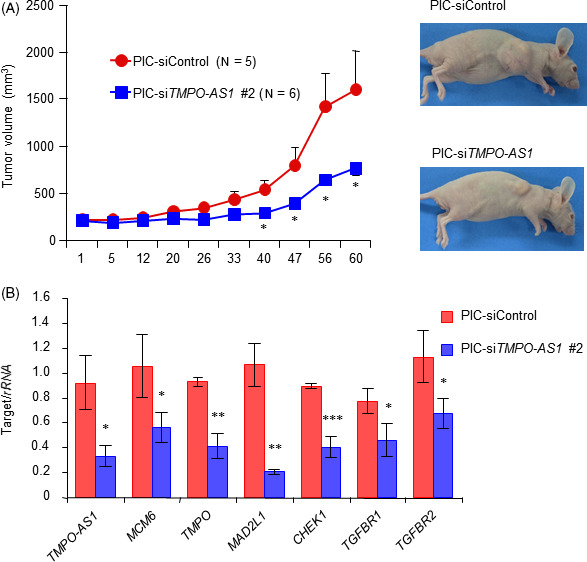
Polyion complex micelle (PIC/m)‐TMPO‐AS1 siRNA treatment suppresses s.c. triple‐negative breast cancer tumor growth. A, B, Progression of MDA‐MB‐231‐derived xenograft tumors treated with PIC/m loaded with siRNAs (PIC/m‐siRNA) in nude mice. PIC/m‐negative control siRNA (siControl) or PIC/m‐siTMPO‐AS1 #2 were i.v. injected into mice inoculated with MDA‐MB‐231 cells through tail veins twice a week (PIC/m‐siControl, n = 5; PIC/m‐siTMPO‐AS1 #2, n = 6). Tumor volumes are presented as mean ± SE (A). Representative photographs of xenografted mice are shown (B). C, TMPO‐AS1, ESR1, MCM6, MAD2L1, CHEK1, TGFBR1, and TGFBR2 levels in tumors treated with PIC/m‐siControl or PIC/m‐siTMPO‐AS #2. Tumors were dissected from mice 60 days after the beginning of siRNA administration. Data are presented as mean fold change ± SD vs siControl (n = 3). *P < .05; **P < .01; ***P < .001
3.6. Lung delivery of DDS‐siTMPO‐AS1 suppresses metastatic tumor growth of TNBC
Because we showed that TMPO‐AS1 is also associated with the migration of TNBC cells, we further examined the therapeutic effect of siRNAs against TMPO‐AS1 based on another lung‐selective DDS nanoball technology (nano‐siTMPO‐AS1), 32 in a lung metastatic model of TNBC cells. Luciferase‐expressing MDA‐MB‐231 cells were i.v. injected into tail veins of NOD/SCID mice. Nano‐siTMPO‐AS1 treatment twice a week for 2 weeks (total of 4 injections) significantly suppressed the viability of MDA‐MB‐231 cells in the lung (Figure 6A,B). To measure viable MDA‐MB‐231 cells in detail, we dissected the lung and measured fluorescence intensities. Fluorescence intensities derived from MDA‐MB‐231 cells stably expressing the luciferase gene were decreased in the nano‐siTMPO‐AS1‐treated group compared with the nano‐siControl‐treated group (Figure 6C). Therefore, TMPO‐AS1 is important for the proliferation and metastasis of TNBC cells, and could serve as a potential therapeutic target of TNBC.
FIGURE 6.
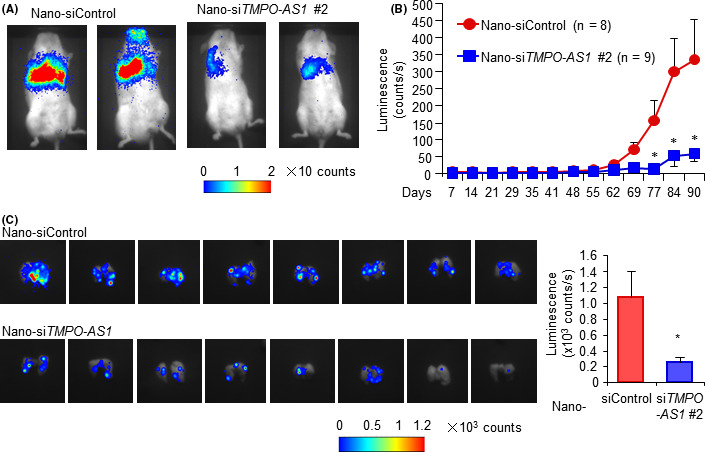
Lung delivery of drug delivery system‐siTMPO‐AS1 suppresses metastatic tumor growth of triple‐negative breast cancer. A, B, Progression of MDA‐MB‐231‐derived xenograft tumors treated with nanoball‐conjugated siRNAs (Nano‐siRNA) in nude mice. Nano‐negative control siRNA (siControl) or nano‐siTMPO‐AS1 #2 were i.v. injected to mice inoculated with MDA‐MB‐231 cells through tail veins twice a week (Nano‐siControl, n = 8; Nano‐siTMPO‐AS1 #2, n = 9). Representative photographs of xenografted mice with fluorescence are shown (A). Tumor volumes are measured with fluorescence and presented as mean ± SE (B). C, MDA‐MB‐231 cells were measured by fluorescence in dissected lung tissues. Data are presented as mean fold change ± SD vs Nano‐siControl (Nano‐siControl, n = 8; Nano‐siTMPO‐AS1 #2, n = 9). *P < .05; **P < .01; ***P < .001
4. DISCUSSION
In the present study, we showed that TMPO‐AS1 is expressed abundantly in TNBC cells as well as in ER‐positive breast cancer cells. Knockdown of TMPO‐AS1 significantly impaired the proliferation, cell cycle progression, and migration of TNBC cells, and promoted apoptosis of TNBC cells. Microarray‐based global expression analysis showed that TMPO‐AS1 is strongly associated with the E2F and TGF‐β signaling pathways. Two distinct DDS‐mediated siRNA treatment methods efficiently impair in vivo tumor growth of primary and metastatic TNBC models in mice.
We previously showed that TMPO‐AS1 is inducible by estrogen treatment 17 ; the present study showed that TMPO‐AS1 is also abundantly expressed in TNBC cells lacking ER expression. TMPO‐AS1 was originally identified as a LAP2α antisense transcript that is inducible by serum treatment. 18 Luciferase assay using putative promoter regions of TMPO/LAP2α and TMPO‐AS1 showed that ectopic E2F3 expression induced both promoter activities. 18 In addition, high TMPO‐AS1 expression was reported in prostate 36 and lung 37 cancers. We speculate that TMPO‐AS1 could be regulated by the E2F signaling pathway in TNBC. Our microarray analysis showed that TMPO‐AS1 knockdown significantly attenuates the E2F signaling pathway, suggesting that TMPO‐AS1 promotes the E2F signaling pathway in TNBC cells. TMPO‐AS1 knockdown decreased the expression of typical E2F target genes, MCM6 and MAD2L1, in both MDA‐MB‐231 and TNBC‐PDC cells. Notably, we previously showed that MCM6 and MAD2L1 were downregulated by ESR1 or TMPO‐AS1 knockdown in ER‐positive breast cancer cells, suggesting that the transcription of these genes is also regulated by estrogen signaling. Overall, TMPO‐AS1 maintains the expression of proliferation‐related factors MCM6 38 and MAD2L1 39 by modulating both estrogen and E2F signaling pathways in breast cancer. Consistently, TMPO‐AS1 overexpression promoted MDA‐MB‐231 cell proliferation and migration, further supporting our findings that TMPO‐AS1 regulates TNBC tumorigenesis.
Intriguingly, we found that TMPO‐AS1 knockdown downregulated its sense gene TMPO. A lung cancer study showed that TMPO‐AS1 contributes to cancer proliferation by regulating TMPO expression. 37 A nuclear membrane‐associated protein, 40 TMPO, or LAP2α, has been reported to be associated with Hutchinson‐Gilford progeria 41 through binding telomeres. A cultured cell study suggested that TMPO could be a therapeutic target for glioblastoma; 42 future studies will reveal the precise molecular mechanism of TMPO in tumorigenesis.
The present study showed that TMPO‐AS1 is also associated with the TGF‐β signaling pathway. The TGF‐β signaling pathway plays an essential role in the development of metastatic phenotype, including cell‐cell interactions and angiogenesis. 33 Transforming growth factor‐β is a cytokine and basically activates its downstream Smad signaling pathway through interaction with TGF‐β receptors. 33 Decreased expression of TGF‐β receptor by siTMPO‐AS1 could attenuate TGF‐β reactivity and signaling, leading to the suppression of breast cancer cell proliferation in mouse models of metastatic tumors. The contribution of TGF‐β signaling in breast cancer metastasis has been previously reported as the overexpression of dominant‐negative mutant TGFBR2 in MDA‐MB‐231 cells or other breast cancer cells that suppresses metastatic phenotype or proliferation of these cells. 43 , 44
In the present study, we identified new TMPO‐AS1 target genes and pathways in TNBC cells. In lung cancer, TMPO‐AS1 stabilizes TMPO RNA and promotes cell proliferation. 27 We have shown that TMPO‐AS1 stabilizes ESR1 mRNA through binding to the 3′‐UTR of ESR1 mRNA in ER‐positive breast cancer. 17 In TNBC, it is likely that TMPO‐AS1 is associated with RNA stability or the decay process; however, further study is required to clarify the precise functions of TMPO‐AS1 in the disease.
Our findings indicate that the inhibition of TMPO‐AS1 could be a useful therapeutic option for TNBC patients, as TMPO‐AS1 targets both the proliferative E2F and metastatic TGF‐β pathways. We used recent powerful DDS technologies, cRGD‐conjugated PIC/m and nanoball, to improve siRNA efficacy through i.v. injection targeting in vivo tumor growth of TNBC cells. Polyion complex micelle‐siTMPO‐AS1 significantly suppressed tumor formation in the s.c. tumor model. The present PIC/m was designed to possess cRGD ligands on its surface, which specifically bind to αvβ3 integrin, for enhanced cancer cell‐binding and accelerated endocytosis. 30 , 31 , 35 Of note, MDA‐MB‐231 cells are reported to express high levels of αvβ3 integrin on their cell surface. 45 Thus, the significant tumor growth inhibition by PIC/m‐siTMPO‐AS1 is probably dependent on higher accumulation of siTMPO‐AS1, which efficiently represses TMPO‐AS1 expression in MDA‐MB‐231 cells.
Moreover, lung‐selective nanoball containing siTMPO‐AS1 notably suppresses tumor formation in a lung metastatic tumor model. Nanoball was able to deliver siRNA into cancer cells through a specific pathway regardless of its anionic charge. 32 Also, Nanoball shows no hematotoxicity or cytotoxicity, unlike in cationic commercial vectors, because of their anionic surface charge. Dendrigraft poly‐L‐lysine consists entirely of lysine, including its central core, and is completely biodegradable, water‐soluble, thermally stable, and nonimmunogenic. 32 , 46 Synthesized γ‐PGA is a biodegradable polymer that causes no inflammatory or immune reactions. 47 Thus, early clinical use of nanoball is expected not only because of efficacy and safety but also biocompatibility. These results suggest that these DDS technologies are useful for exhibiting in vivo gene silencing activity of siTMPO‐AS1 in xenograft tumor models, and could be clinically applied to cancer management.
In conclusion, TMPO‐AS1 plays important roles in the proliferation and migration of TNBC cells, and could be considered as a candidate for molecular targeting therapy in TNBC patients.
DISCLOSURE
The authors have declared that no competing interests exist.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Ms Noriko Sasaki for their technical assistance. This work was in part supported by: the Support Project of Strategic Research Center in Private Universities (to S. Inoue and K. Horie‐Inoue) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan; the Practical Research for Innovative Cancer Control (JP18ck0106194, to K. Ikeda) and the Project for Cancer Research And Therapeutic Evolution (P‐CREATE, JP18cm0106144 to S. Inoue) from Japan Agency for Medical Research and Development, AMED; Grants‐in‐Aid for Scientific Research (B)(17H04205 to K. Horie‐Inoue), for Challenging Exploratory Research (16K15496 to K. Horie‐Inoue), for Young Scientists (B)(17K18061 to Y. Mitobe), and for JSPS fellow (18J00252 to Y. Mitobe) from the Japan Society for the Promotion of Science (JSPS), Japan; and the Takeda Science Foundation (to S. Inoue).
Mitobe Y, Ikeda K, Sato W, et al. Proliferation‐associated long noncoding RNA, TMPO‐AS1, is a potential therapeutic target for triple‐negative breast cancer. Cancer Sci. 2020;111:2440–2450. 10.1111/cas.14498
REFERENCES
- 1. Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev. 2016;17:43‐46. [DOI] [PubMed] [Google Scholar]
- 2. Sharma GN, Dave R, Sanadya J, Sharma P, Sharma KK. Various types and management of breast cancer: an overview. J Adv Pharm Technol Res. 2010;1:109‐126. [PMC free article] [PubMed] [Google Scholar]
- 3. Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321:288‐300. [DOI] [PubMed] [Google Scholar]
- 4. Zwart W, Terra H, Linn SC, Schagen SB. Cognitive effects of endocrine therapy for breast cancer: keep calm and carry on? Nat Rev Clin Oncol. 2015;12:597‐606. [DOI] [PubMed] [Google Scholar]
- 5. Bilous M, Dowsett M, Hanna W, et al. Current perspectives on HER2 testing: a review of national testing guidelines. Mod Pathol. 2003;16:173‐182. [DOI] [PubMed] [Google Scholar]
- 6. Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple‐negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. 2010;7:683‐692. [DOI] [PubMed] [Google Scholar]
- 7. Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple‐negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13:674‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kotzin JJ, Spencer SP, McCright SJ, et al. The long non‐coding RNA Morrbid regulates Bim and short‐lived myeloid cell lifespan. Nature. 2016;537:239‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Faghihi MA, Modarresi F, Khalil AM, et al. Expression of a noncoding RNA is elevated in Alzheimer's disease and drives rapid feed‐forward regulation of beta‐secretase. Nat Med. 2008;14:723‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mitobe Y, Takayama KI, Horie‐Inoue K, Inoue S. Prostate cancer‐associated lncRNAs. Cancer Lett. 2018;418:159‐166. [DOI] [PubMed] [Google Scholar]
- 11. Kopp F, Mendell JT. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell. 2018;172:393‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29:452‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin A, Li C, Xing Z, et al. The LINK‐A lncRNA activates normoxic HIF1α signalling in triple‐negative breast cancer. Nat Cell Biol. 2016;18:213‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu Q, Ye Y, Chan L‐C, et al. Oncogenic lncRNA downregulates cancer cell antigen presentation and intrinsic tumor suppression. Nat Immunol. 2019;20:835‐851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li C, Wang S, Xing Z, et al. A ROR1‐HER3‐lncRNA signalling axis modulates the Hippo‐YAP pathway to regulate bone metastasis. Nat Cell Biol. 2017;19:106‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mitobe Y, Ikeda K, Suzuki T, et al. ESR1‐stabilizing long noncoding RNA TMPO‐AS1 promotes hormone‐refractory breast cancer progression. Mol Cell Biol. 2019;39:e00261‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parise P, Finocchiaro G, Masciadri B, et al. Lap2alpha expression is controlled by E2F and deregulated in various human tumors. Cell Cycle. 2006;5:1331‐1341. [DOI] [PubMed] [Google Scholar]
- 19. Devi GR. siRNA‐based approaches in cancer therapy. Cancer Gene Ther. 2006;13:819‐829. [DOI] [PubMed] [Google Scholar]
- 20. Singh A, Trivedi P, Jain NK. Advances in siRNA delivery in cancer therapy. Artif Cells Nanomed Biotechnol. 2018;46:274‐283. [DOI] [PubMed] [Google Scholar]
- 21. Barata P, Sood AK, Hong DS. RNA‐targeted therapeutics in cancer clinical trials: Current status and future directions. Cancer Treat Rev. 2016;50:35‐47. [DOI] [PubMed] [Google Scholar]
- 22. Cabral H, Miyata K, Osada K, Kataoka K. Block copolymer micelles in nanomedicine applications. Chem Rev. 2018;118:6844‐6892. [DOI] [PubMed] [Google Scholar]
- 23. Tominaga K, Shimamura T, Kimura N, et al. Addiction to the IGF2‐ID1‐IGF2 circuit for maintenance of the breast cancer stem‐like cells. Oncogene. 2017;36:1276‐1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tominaga K, Minato H, Murayama T, et al. Semaphorin signaling via MICAL3 induces symmetric cell division to expand breast cancer stem‐like cells. Proc Natl Acad Sci USA. 2019;116:625‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. The Cancer Genome Atlas Network . Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li J, Han L, Roebuck P, et al. TANRIC: an interactive open platform to explore the function of lncRNAs in cancer. Cancer Res. 2015;75:3728‐3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Christie RJ, Matsumoto YU, Miyata K, et al. Targeted polymeric micelles for siRNA treatment of experimental cancer by intravenous injection. ACS Nano. 2012;6:5174‐5189. [DOI] [PubMed] [Google Scholar]
- 31. Nishida H, Matsumoto Y, Kawana K, et al. Systemic delivery of siRNA by actively targeted polyion complex micelles for silencing the E6 and E7 human papillomavirus oncogenes. J Control Release. 2016;231:29‐37. [DOI] [PubMed] [Google Scholar]
- 32. Kodama Y, Kuramoto H, Mieda Y, et al. Application of biodegradable dendrigraft poly‐l‐lysine to a small interfering RNA delivery system. J Drug Target. 2017;25:49‐57. [DOI] [PubMed] [Google Scholar]
- 33. Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bellomo C, Caja L, Moustakas A. Transforming growth factor β as regulator of cancer stemness and metastasis. Br J Cancer. 2016;115:761‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Katsushima K, Natsume A, Ohka F, et al. Targeting the Notch‐regulated non‐coding RNA TUG1 for glioma treatment. Nat Commun. 2016;7:13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang W, Su X, Yan W, et al. Overexpression of AR‐regulated lncRNA TMPO‐AS1 correlates with tumor progression and poor prognosis in prostate cancer. Prostate. 2018;78:1248‐1261. [DOI] [PubMed] [Google Scholar]
- 37. Qin Z, Zheng X, Fang Y. Long noncoding RNA TMPO‐AS1 promotes progression of non‐small cell lung cancer through regulating its natural antisense transcript TMPO. Biochem Biophys Res Commun. 2019;516:486‐493. [DOI] [PubMed] [Google Scholar]
- 38. Issac MSM, Yousef E, Tahir MR, Gaboury LA. MCM2, MCM4, and MCM6 in breast cancer: clinical utility in diagnosis and prognosis. Neoplasia. 2019;21:1015‐1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yuan B, Xu Y, Woo JH, et al. Increased expression of mitotic checkpoint genes in breast cancer cells with chromosomal instability. Clin Cancer Res. 2006;12:405‐410. [DOI] [PubMed] [Google Scholar]
- 40. Dechat T, Vlcek S, Foisner R. Review: lamina‐associated polypeptide 2 isoforms and related proteins in cell cycle‐dependent nuclear structure dynamics. J Struct Biol. 2000;129:335‐345. [DOI] [PubMed] [Google Scholar]
- 41. Chojnowski A, Ong PF, Wong ESM, et al. Progerin reduces LAP2α‐telomere association in Hutchinson‐Gilford progeria. Elife. 2015;4:e07759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang L, Wang G, Chen S, et al. Depletion of thymopoietin inhibits proliferation and induces cell cycle arrest/apoptosis in glioblastoma cells. World J Surg Oncol. 2016;14:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yin JJ, Selander K, Chirgwin JM, et al. TGF‐beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tang B, Vu M, Booker T, et al. TGF‐beta switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J Clin Invest. 2003;112:1116‐1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bauer K, Mierke C, Behrens J. Expression profiling reveals genes associated with transendothelial migration of tumor cells: a functional role for alphavbeta3 integrin. Int J Cancer. 2007;121:1910‐1918. [DOI] [PubMed] [Google Scholar]
- 46. Kodama Y, Nakamura T, Kurosaki T, et al. Biodegradable nanoparticles composed of dendrigraft poly‐L‐lysine for gene delivery. Eur J Pharm Biopharm. 2014;87:472‐479. [DOI] [PubMed] [Google Scholar]
- 47. Ye H, Jin L, Hu R, et al. Poly(gamma, L‐glutamic acid)‐cisplatin conjugate effectively inhibits human breast tumor xenografted in nude mice. Biomaterials. 2006;27:5958‐5965. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


