Table 4.
Experimental data for compounds 5–7.
| compound | 1H NMR multiplicity of 2-CHa,b | E≠cis-to-transc kJ mol−1 | logP | ||||||
| in CD2Cl2 | in D2O | in D2O | in C6D6 | ||||||
| 1J, Hz | 2J, Hz | C4- | 1J, Hz | 2J, Hz | C4- | ||||
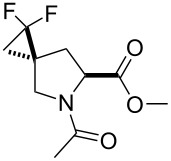 5 |
Ktrans/cis = 3.13 ± 0.07 | Ktrans/cis = 3.94 ± 0.03 | 82.4 ± 0.4 | 73.4 ± 0.1 | +0.18 ± 0.03 | ||||
| 8.8 (8.7) |
5.0 (2.8) |
mixd (endo) |
8.8 (8.8) |
5.4 (2.3) |
mixd (endo) |
Δwater/benzene = 9.0 | |||
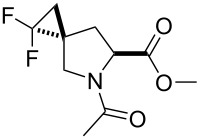 6 |
Ktrans/cis = 2.47 ± 0.02 | Ktrans/cis = 3.15 ± 0.05 | 84.5 ± 0.2 | 74.2 ± 0.2 | +0.03 ± 0.02 | ||||
| 8.9 (8.5) |
1.8 (1.0) |
endo (endo) |
9.1 (8.6) |
1.2 (n.d.)e |
endo (endo) |
Δwater/benzene = 10.3 | |||
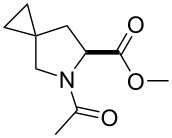 7 |
Ktrans/cis = 3.35 ± 0.02 | Ktrans/cis = 3.92 ± 0.04 | 85.6 ± 1.1 | 76.3 ± 0.1 | +0.19 ± 0.03 | ||||
| 8.6 (8.5) |
4.4 (2.4) |
mixd (endo) |
8.8 (8.4) |
3.6 (n.d.)e |
mixd (endo) |
Δwater/benzene = 9.5 | |||
aRead out from 1D 1H NMR spectra recorded at 700 MHz frequency at 298 K; bthe s-trans (major) amide rotamer data is shown first; the results in parentheses are for the s-cis (minor) amide rotamer; cmeasured by 1H EXSY NMR at 298 K for C6D6 and 310 K for D2O; dmix = a mixture of two conformations; en.d. not detected.
