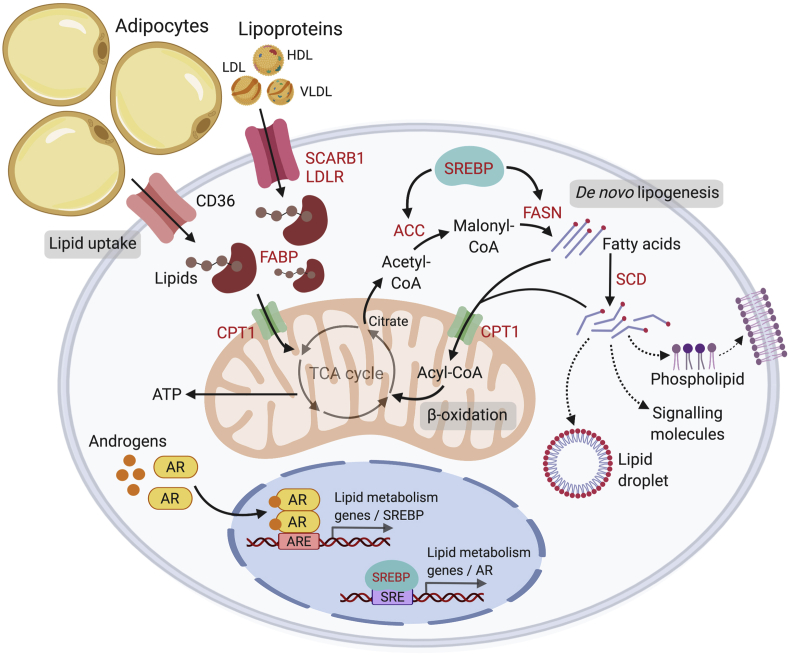
Figure 2.
Overview of lipid metabolism in prostate cancer. Androgens regulate a number of lipid metabolic pathways in prostate cancer cells, including lipid uptake, biosynthesis and degradation. The AR directly (via binding of AR to the ARE) or indirectly (via activation of SREBP) regulates key lipid metabolic genes. Lipids can be derived from lipolysis of adipocytes or from circulating lipoproteins (LDL, HDL, VLDL). Alternatively, lipids can be synthesized endogenously by the cells via enzymes ACC and FASN. Androgens up-regulate lipid transporters that transport lipids or free fatty acids to the mitochondria for fatty acid oxidation (β-oxidation) or de novo lipogenesis. Free fatty acids are shuttled into the mitochondria as Acyl-CoAs by coupling with CPT1 (a mitochondrial transporter); a cyclical series of reactions result in the shortening of Acyl-CoA and the production of energy in the form of ATP via the TCA cycle. Citrate produced from the TCA cycle may also be cleaved to produce Acetyl-CoA, which is a precursor for de novo lipogenesis in the cytosol. Synthesized fatty acids may be further modified (i.e. degree of saturation via SCD) to generate a variety of lipids such as phospholipids that compose the plasma membranes, to promote lipid droplet production or to act as oncogenic signalling molecules to drive disease progression. Words in red = androgen-regulated genes. ARE, androgen response element; SRE, SREBP response element; AR, androgen receptor; LDL, low density lipoprotein; HDL, high density lipoprotein; VLDL, very low density lipoprotein; FASN, fatty acid biosynthesis; TCA, tricarboxylic acid cycle; SCD, stearoyl-CoA-desaturase; SREBP, sterol response element binding protein. Created with BioRender.com.