Figure 1.
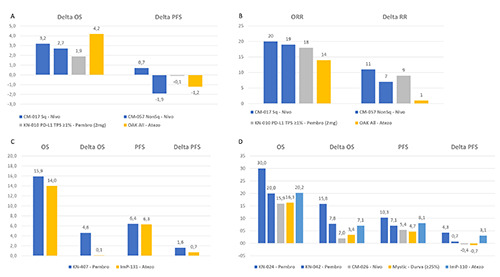
Indirect comparisons between anti-PD1 and anti-PD-L1 exploring possible clinical efficacy differences: A, indirect comparison of difference (Delta) in median months of OS and PFS between anti-PD1 and anti-PD-L1 agents and chemotherapy in the secondand beyond line treatment of aNSCLC by available phase III randomized trials; B, indirect comparison of ORR percentage between anti-PD1 and anti-PD-L1 agents and of gain in ORR (Delta) percentage between them and chemotherapy in the second- and beyond line treatment of aNSCLC by available phase III randomized trials; C, indirect comparison of median months of OS and PFS between anti-PD1 and anti-PD-L1 agents plus chemotherapy and of gain in OS and PFS (Delta) months between them and chemotherapy alone in the first-line treatment of squamous aNSCLC by available phase III randomized trials; D, indirect comparison of median months of OS and PFS between anti-PD1 and anti-PD-L1 agents and of gain in OS and PFS (Delta) months between them and chemotherapy in the first-line treatment of PD-L1 high aNSCLC by available phase III randomized trials. aNSCLC, advanced nonsmall-cell lung cancer; ADCC, antibody-dependent cell-mediated cytotoxicity (ADCC); Atezo, atezolizumab; CM, checkmate; G, grade; KN, keynote; Ig, immunoglobulin; ImP, ImPower; moAbs, monoclonal antibodies; Nivo, nivolumab; ORR, overall response rate; OS, overall survival; PD1, programmed cell death 1; PD-L1, programmed cell death ligand 1; Pembro, pembrolizumab; PFS, progressionfree survival; Sq, squamous.
