Table 1.
Chemical drug in clinical against COVID-19.
| Compounds | Chemical structures | Drug Target against COVID-19 | EC50 | Severe adverse effects |
|---|---|---|---|---|
| Lopinavir/ritonavir (LPV/r) | 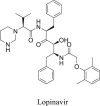 |
3CLprp | 26.63 μM | No obvious severe adverse reports |
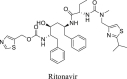 |
CYP3A4 | >100 μM | ||
| Chloroquine (CQ) | 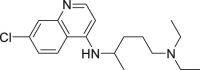 |
inhibit viral membrane fusion process | 1.13 μM | Retinopathy, cardiomyopathy, and neuromyopathy |
| Hydroxychloroquine(HCQ) | 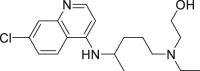 |
inhibit viral membrane fusion process | 0.72 μM | Retinopathy, cardiomyopathy, and neuromyopathy |
| Arbidol (Umifenovir) | 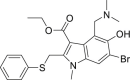 |
Spike glycoprotein | 4.11 μM | Hepatic insufficiency |
| Remdesivir (GS-5734) | 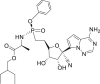 |
RdRp | 0.77 μM | No obvious severe adverse reports |
| Favipiravir (T-705) | 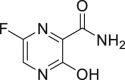 |
RdRp | 61.88 μM | Teratogenicity and embryotoxicity |
| Baricitinib | 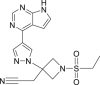 |
JAK | – | A risk of reactivation of latent infections |
| Ribavirin | 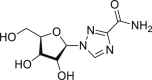 |
RdRp | >100 μM | Hemolytic anemia and teratogenic |
| Galidesivir (BCX4430) | 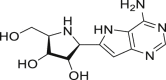 |
RdRp | >100 μM | No obvious severe adverse reports |
| Darunavir | 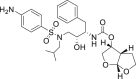 |
3CLprp | – | Hepatic insufficiency |
| Oseltamivir | 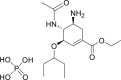 |
unknown | >100 μM | Severe rash and hemorrhagic colitis |
| Camostat mesylate | 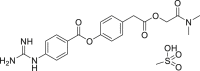 |
TMPRSS2 | – | No obvious severe adverse reports |
| 11a | 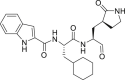 |
3CLprp | 0.53 μM | Unknown |
| 11b | 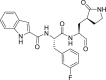 |
3CLprp | 0.72 μM | Unknown |
| 13b | 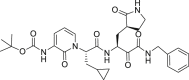 |
3CLprp | 4–5 μM | Unknown |
| N3 | 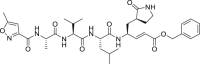 |
3CLprp | 16.77 μM | Unknown |
