Fig. 2.
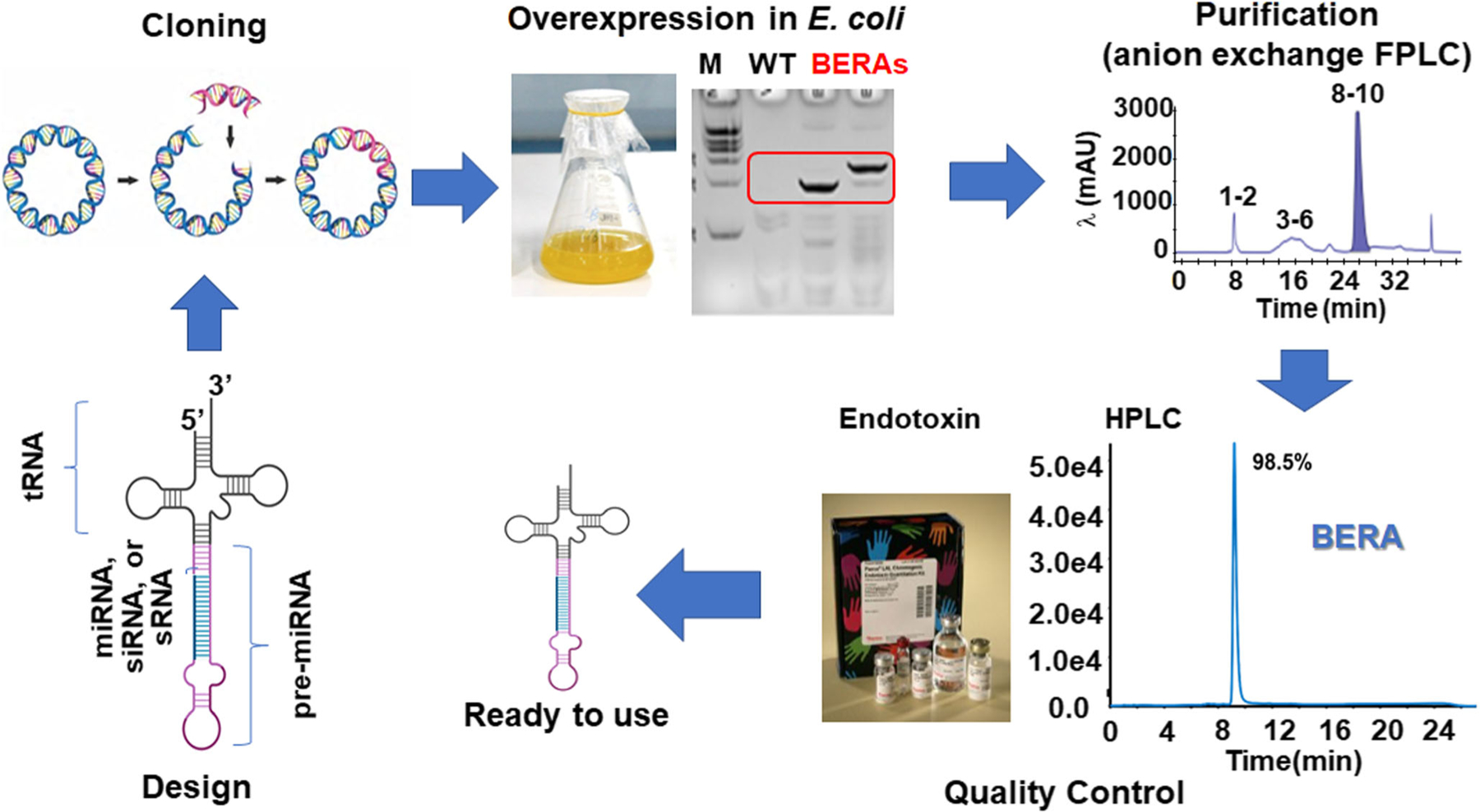
Streamlined workflow for fermentation production of biologic RNAi agent or BERA using the tRNA/pre-miRNA carrier. After a BERA is designed to accommodate the miRNA/siRNA/sRNA of interest, the coding sequence is cloned into a vector. Overexpression of the target BERA is easily verified by gel electrophoresis of total bacterial RNA. After the BERA is purified (e.g., with anion exchange FPLC method), its purity and endotoxin level are verified by HPLC method and commercially available endotoxin assay kits, respectively. These BERAs should better recapitulate the properties of natural RNAs as both are produced and folded in living cells. Therefore, BERAs are a novel class of biologic RNA molecules for research and development
