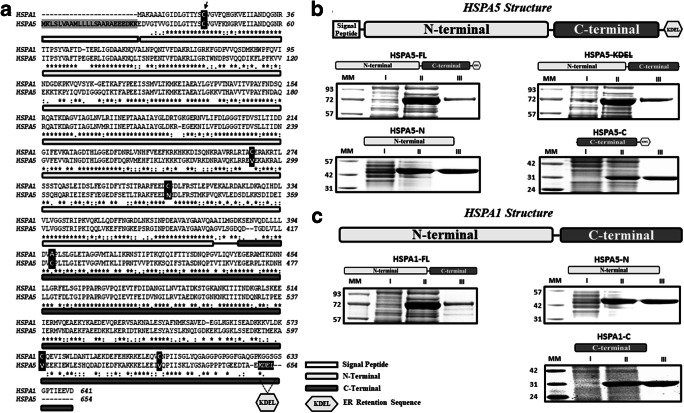
Fig. 1.
HSPA5 and HSPA1 share a high identity, and the proteins were isolated with high purity. a The global alignment between HSPA5 and HSPA1 indicates 64% of identity, which is considered high. The N-terminal of these proteins presents approximately 68% (flat gray rectangle), and the C-terminal presents 58% of identity (dark gray flat rectangle). Cysteine groups are indicated by black boxes, two for HSPA5 and five for HSPA1. The ER translocation signal peptide is displayed by the shadow area, and the ER retention signal (KDEL) is indicated by a hexagon. b HSPA5-FL, HSPA5-KDEL, HSPA5-N, and HSPA5-C. c HSPA1-FL, HSPA1-N, and HSPA1-C. Proteins were purified as described in Methods. All steps for the production and isolation process were visualized by LDS-PAGE and Coomassie blue staining. All proteins were obtained with more than 92% purity.