Graphical abstract
The clinical efficacy of glucocorticoid therapy on the treatment of patients with Coronavirus Disease 2019 (COVID-19) pneumonia was investigated. This retrospective study showed glucocorticoid therapy did not significantly influence the clinical course of COVID-19 pneumonia, including imaging progress and the time duration for negative transformation of nucleic acid, and glucocorticoid therapy did not significantly influence the outcomes nor the adverse events of COVID-19 pneumonia.
Keywords: COVID-19, Glucocorticoid therapy, Clinical course
Highlights
-
•
Glucocorticoid therapy did not significantly influence the clinical course of Coronavirus Disease 2019 (COVID-19) pneumonia, including imaging progress and the time duration for negative transformation of nucleic acid.
-
•
Glucocorticoid therapy did not significantly influence the adverse events of COVID-19 pneumonia.
-
•
Glucocorticoid therapy did not significantly influence the outcomes of COVID-19 pneumonia.
Abstract
The aim of the present study was to identify the clinical efficacy of glucocorticoid therapy on the treatment of patients with Coronavirus Disease 2019 (COVID-19) pneumonia. Clinical and laboratory parameters were collected from 308 patients with COVID-19 pneumonia from the fever clinic of Wuhan Pulmonary Hospital (Wuhan City, Hubei Province, China) between January 14, 2020 and February 9, 2020, of which 216 patients received low-dose (equivalent of methylprednisolone 0.75–1.5 mg/kg/d) glucocorticoid treatment. The effect of glucocorticoid on imaging progress, adverse events, nucleic acid results and the outcomes were investigated. Lymphocyte count and C-reactive protein (CRP) significantly differed between the glucocorticoid therapy and non-glucocorticoid therapy groups. Compared with the non-glucocorticoid therapy group, glucocorticoid therapy did not significantly influence the clinical course of COVID-19 pneumonia, including imaging progress and the time duration for negative transformation of nucleic acid. Glucocorticoid therapy did not significantly influence the outcomes nor the adverse events of COVID-19 pneumonia. For the treatment of COVID-19 pneumonia, systemic and in-depth investigation is needed to determine the timing and dosage of glucocorticoids needed to inhibit overwhelming inflammatory response and not the protective immune response to COVID-19 pneumonia.
1. Introduction
Since December 2019, Wuhan, China, has experienced an outbreak of COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The virus easily invades the lungs after infecting the human body, and in severe cases, it can quickly progress to acute respiratory distress syndrome [[1], [2], [3], [4]]. Based on the current epidemiological investigation, the incubation period was 1–14 days, mostly 3–7 days [5], with fever, dry cough and fatigue as the main manifestations, and some patients with stuffy nose, runny nose, sore throat and diarrhoea [6,7]. At present, with the progress of the pandemic, confirmed and clinically diagnosed cases continue to rise, and the death toll is also increasing [8,9]. However, the drug treatment of the disease is still in the exploratory stage, and there is still a lack of specific anti-COVID-19 pneumonia drugs. According to the recommendation of the Chinese management guideline for COVID-19 pneumonia (version 5.0) [5] issued by the National Health Commission of the People’s Republic of China, using aerosol inhalation of α-interferon, oral administration of lopinavir/ritonavir, intravenous infusion of ribavirin, or oral antiviral therapy of abidol and chloroquine phosphate to avoid blind or inappropriate use of antimicrobials, especially the combined use of broad-spectrum antimicrobials, were recommended. At present, the use of glucocorticoid is still under discussion and needs to be used with caution.
To gain a better understanding of the status, function and rational use of glucocorticoid in the treatment of COVID-19 pneumonia, we summarised outpatient case information and analysed the baseline clinical characteristics of glucocorticoid therapy and non-glucocorticoid therapy groups, and the effect of glucocorticoid therapy on clinical course, adverse events and the outcomes were investigated. The present work presented the results of the single medical center in China.
2. Methods
2.1. Patients and data collection
This retrospective study was approved by the medical ethics committee of Wuhan Pulmonary Hospital, and the approval number was Wuhan Pulmonary Hospital Ethics (2020) No. 28. We collected clinical data of all patients who visited the fever clinic of Wuhan Pulmonary Hospital from January 14 to February 9, 2020. Among these patients, there were 18 cases of upper respiratory tract infection, 53 cases of non-viral pneumonia, 30 cases of tuberculosis, 2 cases of non-tuberculous mycobacteria and 1167 cases of COVID-19 pneumonia. Among the COVID-19 pneumonia cases, 321 patients were directly admitted to the hospital, and 538 patients had less than 2 outpatient visits or less than 3 days of outpatient treatment. We excluded these patients, and finally 308 patients with COVID-19 pneumonia were included in the present study. These patients had lung lesions before or after treatment. The follow-up deadline of subjects in both groups was March 14. The confirmed and clinical diagnosis of COVID-19 pneumonia was based on the Chinese management guideline for COVID-19 pneumonia (version 5.0) [5] issued by the National Health Commission of the People’s Republic of China. The collected information mainly included demographic data, clinical symptoms and characteristics, adverse events, chest imaging results and nucleic acid results. During the outpatient consultation, the comorbidity inquiry content included whether they were combined with diabetes, hypertension, pulmonary tuberculosis, cardiovascular disease and chronic obstructive pulmonary disease (COPD), and other disease information was provided by patients themselves.
2.2. SARS-CoV-2 detection in swab samples
Throat swab samples of patients were collected and stored separately in 5 mL of virus preservation solution, and virus RNA was extracted over 24 h using a Tianlong PANA9600 automatic nucleic acid extraction system with ready-made reagents. The open reading frame 1ab (ORF1ab) and nucleocapsid protein (N) genes were simultaneously tested using a commercial real-time reverse transcription polymerase chain reaction (RT-PCR) kit from DAAN GENE (Guangzhou, China). RT-PCR assay was performed on a Tianlong Gentier 96E real-time PCR system. The steps were as follows: incubation at 50℃ for 15 min, pre-denaturation at 95℃ for 15 min, denaturation at 94℃ for 45 cycles for 15 s, and extension at 55℃ for 45 s. A cycle threshold value (Ct-value) of 40 or more for the two genes was defined as negative, and a Ct-value less than 40 for the two genes was defined as positive. Samples with a single Ct-value less than 40 need to be confirmed by retesting [10].
2.3. Statistical analysis
All statistical analysis was performed using SPSS 22.0 version software. The categorical variables were demonstrated by frequency rates and percentages. The proportion of categorical variables was compared using the χ2 test. Fisher's exact test was used when data is limited. Continuous variables were described using mean, median and interquartile range (IQR) values. Means for continuous variables were compared using independent group t tests when the data were normally distributed; otherwise, the Mann-Whitney test was used.
3. Results
3.1. Patient characteristics
A total of 308 patients were included. The median age was 53 years old (IQR, 42−62), ranging from 18 years to 80 years, and 145 of the 308 patients (47.1 %) were male. The clinical, radiological and laboratory data in our cohort is summarised in Table 1 . Comorbidities were present in many COVID-19 pneumonia patients, with the highest probability of hypertension and diabetes. The most common symptoms at onset of illness were fever (260 [84.4 %]), dry cough (184 [59.7 %]), fatigue (120 [39.0 %]), expectoration (84 [27.3 %]) and headache (84 [27.3 %]). All of the 308 patients enrolled showed pathological changes in the chest computed tomography (CT) scan. The first CT imaging data of patients demonstrated that 196 patients (60.4 %) had pathological changes in more than 2 lung segments. The most common feature of CT change was ground-glass opacity, followed by consolidation and fibrosis (Table 1). However, compared with patients who did not receive glucocorticoid treatment (n = 92), patients who required glucocorticoid treatment (n = 216) were older (median age, 54 years [IQR, 44−63] vs 48 years [IQR, 39−60]; p = 0.03). Compared with the non-glucocorticoid treatment patients, the patients receiving glucocorticoid therapy were more likely to have fever. Patients in the glucocorticoid therapy group had significantly lower SaO2 than their counterparts (p = 0.003, Table 1). The frequency of people who had pathological changes in more than 2 lung segments of the first CT imaging was higher in the glucocorticoid treatment group than their counterparts (146 [67.6 %] vs 50 [54.3 %], p = 0.03). The rates of simple consolidation shadow in the glucocorticoid treatment group was significantly higher than those in the non-glucocorticoid group (41 [19.0 %] vs 9 [9.8 %], p = 0.04). White blood cell (WBC) count did not differ between patients who received glucocorticoid therapy and those who did not receive glucocorticoid therapy. There were numerous differences in laboratory findings between the glucocorticoid treatment group and the non-glucocorticoid group (Table 1), including lower lymphocyte count (p < 0.001) and higher CRP (p < 0.001) in the glucocorticoid treatment group.
Table 1.
Baseline clinical characteristics of COVID-19 pneumonia patients.
| Variables | Total (N = 308) | Glucocorticoid therapy group (N = 216) | Non-glucocorticoid group (N = 92) | P value |
|---|---|---|---|---|
| Age (yr), median (Q1,Q3) | 53 (42−62) | 54 (44−63) | 48 (39−60) | 0.03 |
| Sex, n(%) | ||||
| Male | 145 (47.1) | 102 (47.2) | 43 (46.7) | 0.94 |
| Female | 163 (52.9) | 114 (52.8) | 49 (53.3) | |
| Disease severity status, n(%) | ||||
| General | 284 (92.2) | 195 (90.3) | 89 (96.7) | 0.05 |
| Severe | 24 (7.8) | 21 (9.7) | 3 (3.3) | |
| Disease classification, n(%) | ||||
| Confirmed case | 190 (61.7) | 137 (63.4) | 53 (57.6) | 0.37 |
| Clinically diagnosed case | 118 (38.3) | 79 (36.6) | 39 (42.4) | |
| Signs and symptoms, n(%) | ||||
| Fever (temperature≥37.3 °C) | 260 (84.4) | 191 (88.4) | 69 (75.0) | 0.003 |
| Dry cough | 184 (59.7) | 129 (59.7) | 55 (59.8) | 0.99 |
| Expectoration | 84 (27.3) | 61 (19.8) | 23 (7.5) | 0.56 |
| Fatigue | 120 (39.0) | 87 (40.3) | 33 (35.9) | 0.47 |
| Headache | 84 (27.3) | 64 (20.8) | 20 (6.5) | 0.15 |
| Comorbidities, n(%) | ||||
| Hypertension | 39 (12.7) | 31 (14.4) | 8 (8.7) | 0.17 |
| Diabetes | 23 (7.5) | 20 (9.3) | 3 (3.3) | 0.07 |
| Pulmonary tuberculosis | 14 (4.5) | 8 (3.7) | 6 (6.5) | 0.43 |
| Cardiovascular disease | 7 (2.3) | 4 (1.9) | 3 (3.3) | 0.73 |
| COPD | 4 (1.3) | 3 (1.4) | 1 (1.1) | 0.74 |
| Blood oxygen saturation(%), median (Q1,Q3) | 97 (96−98) | 96 (95−98) | 97 (96−98) | 0.003 |
| The range of lesions involved in the first image, n(%) | ||||
| ≤ 2 lung lobes | 112 (36.4) | 70 (32.4) | 42 (45.7) | 0.03 |
| >2 lung lobes | 196 (60.4) | 146 (67.6) | 50 (54.3) | |
| First image features, n(%) | ||||
| Ground-glass opacity | 282 (91.6) | 201 (93.1) | 81 (88.0) | 0.15 |
| Consolidation | 50 (16.2) | 41 (19.0) | 9 (9.8) | 0.04 |
| Interstitial changes | 23 (7.5) | 17 (7.9) | 6 (6.5) | 0.68 |
| Fibrosis | 27 (8.8) | 19 (8.8) | 8 (8.7) | 0.98 |
| Pleural effusion | 6 (1.9) | 6 (2.8) | 0 | 0.24 |
| Laboratory inspection | ||||
| White blood cell count, ×109/L, median (Q1,Q3) | 5.4 (4.4−6.9) | 5.5 (4.4−7.1) | 5.4 (4.4−6.5) | 0.65 |
| <4, n(%) | 53 (17.6) | 39 (18.5) | 14 (15.6) | 0.54 |
| 4–10, n(%) | 235 (78.1) | 161 (76.3) | 74 (82.2) | |
| >10, n(%) | 13 (4.3) | 11 (5.2) | 2 (2.2) | |
| Lymphocyte count, ×109/L, median (Q1,Q3) | 1.1 (0.8−1.5) | 1.0 (0.7−1.3) | 1.3 (0.9−1.8) | <0.0001 |
| <1.1, n(%) | 157 (52.2) | 125 (59.2) | 32 (35.6) | 0.0002 |
| C-reactive protein, mg/L, median (Q1,Q3) | 16.1 (4.9−37.4) | 21.0 (7.9−43.5) | 5.6 (0.9−23.3) | <0.0001 |
| >5, n(%) | 219 (72.8) | 173 (82.0) | 46 (51.1) | <0.0001 |
3.2. Glucocorticoid use
Due to incomplete follow-up data and the presence of missing patients, we included a final total of 104 patients for analysis of subsequent outcomes, including 58 confirmed patients and 46 clinically diagnosed patients. Methylprednisolone was the most frequently administered glucocorticoid (84/86 [97.7 %]), followed by prednisolone (15/86 [17.4 %]) (Table 2 ). The most common and maximum equivalent of methylprednisolone doses were 0.75 mg/kg/d and 1.5 mg/kg/d respectively. Among COVID-19 pneumonia patients, glucocorticoid therapy was started at a median of 7 (IQR, 6−10) days from onset of illness, the median of glucocorticoid total equivalent dose was 200 (160, 280) mg with a median duration of 6 (IQR,4−8) days.
Table 2.
Glucocorticoid among COVID-19 pneumonia patients (n = 86).
| Medication Variable | Patients (n = 86) |
|---|---|
| Duration between onset of illness and glucocorticoid initiation (d), median (Q1,Q3) | 7 (6−10) |
| Methylprednisolone, n(%) | 84/86 (97.7) |
| Prednisolone, n(%) | 15/86 (17.4) |
| Duration of glucocorticoid (d), median (Q1,Q3) | 6 (4−8) |
| Dose,glucocorticoid total equivalents (mg), median (Q1,Q3) | 200 (160−280) |
3.3. Clinical course and outcomes
The clinical course of the COVID-19 pneumonia patients was presented in Table 3 . Among these patients, 86 (82.6 %) received glucocorticoid treatment, and 18 (17.4 %) did not receive glucocorticoid therapy. Frequency of visit (7, IQR 5−8 days; 5, IQR 4−6 days; p = 0.06), duration of consultation (17, IQR 12−32 days; 20, IQR 13−30 days; p = 0.73) and duration of fever (8, IQR 7−11 days; 8, IQR 6−11 days; p = 0.19) did not differ between patients who received glucocorticoid therapy and patients who did not receive glucocorticoid therapy. No significant differences were observed as to the amount of time required for body temperature to return to normal between the glucocorticoid therapy group and its counterparts (4, IQR 2–7 days; 5, IQR 2−6 days; p = 0.68).
Table 3.
Clinical course and outcomes for COVID-19 pneumonia patients of the glucocorticoid therapy and the non-glucocorticoid therapy groups.
| Variables | Total (N = 104) | Glucocorticoid therapy group (N = 86) | Non-glucocorticoid group (N = 18) | P value |
|---|---|---|---|---|
| Clinical course | ||||
| Frequency of visit, median (Q1,Q3) | 6 (5−8) | 7 (5−8) | 5 (4−6) | 0.06 |
| Duration of consultation (d), median (Q1,Q3) | 19 (12−31) | 17 (12−32) | 20 (13−30) | 0.73 |
| Duration of fever (d), median (Q1,Q3) | 7 (3−11) | 8 (7−11) | 8 (6−11) | 0.19 |
| Duration of body temperature returned to normal (d), median (Q1,Q3) | 4 (2−7) | 4 (2−7) | 5 (2−6) | 0.68 |
| CT image (d), median (Q1,Q3) | ||||
| Duration from first CT examination to the first discovery of radiographic progression | 4 (3−5) | 4 (3−6) | 3 (3−4) | 0.53 |
| Duration from the first discovery of radiographic progression to the first discovery of pulmonary absorption | 6 (4−9) | 6 (4−9) | 6 (5−6) | 0.77 |
| Duration from first CT examination to the first discovery of pulmonary absorption | 11 (8−15) | 11 (9−14) | 11 (8−15) | 0.87 |
| Duration from treatment to residual lung lesions | 26 (22−27) | 26 (22−27) | 20 | N/A |
| Nucleic acid result | ||||
| Negative transformation of nucleic acid, n(%) | 104 (100) | 86 (100) | 18 (100) | N/A |
| Duration for negative transformation of nucleic acid(d), median (Q1,Q3) | 20 (16−23) | 18 (15−23) | 20 (18−23) | 0.55 |
| Adverse events, n(%) | ||||
| Random blood glucose elevation | 21 (20.2) | 19 (22.1) | 2 (11.1) | 0.46 |
| Hypokalemia | 24 (23.1) | 21 (24.4) | 3 (16.7) | 0.69 |
| Outcomes, n(%) | ||||
| Cure | 88 (84.6) | 73 (84.9) | 15 (83.3) | 0.85 |
| In-hosptial | 16 (15.4) | 13 (15.1) | 3 (16.7) | |
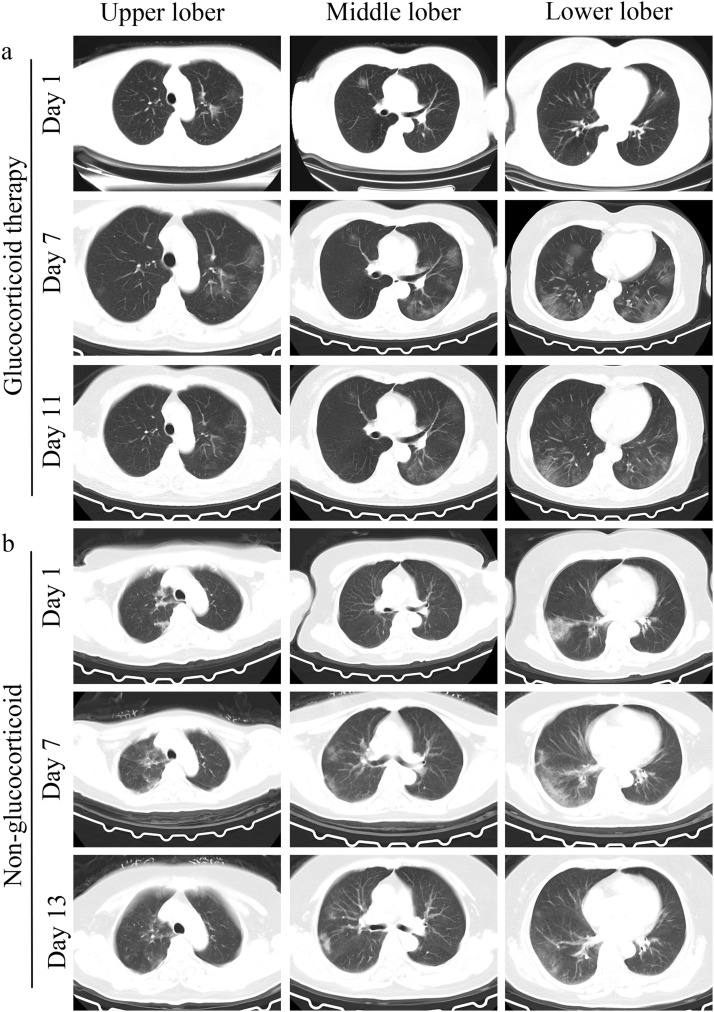
There was often a certain time interval from symptom initiation to the hospital consultation. Among COVID-19 pneumonia patients, the median interval between the first CT examination and the first discovery of radiographic progression was 4 (IQR, 3−6) days for the glucocorticoid therapy group, and there was no significant difference as to the duration from first CT examination to the first discovery of pulmonary absorption between the glucocorticoid therapy and non-glucocorticoid therapy groups (11, IQR 9−14 days; 11, IQR 8−15 days; p = 0.87). The median duration from the first discovery of radiographic progression to the first discovery of pulmonary absorption was 6 (IQR, 4−9) days for total patients. No significant differences as to the duration time from the first discovery of radiographic progression to the first discovery of pulmonary absorption between two groups (6, IQR 4−9 days; 6, IQR 5−6 days; p = 0.77) were observed. As shown in Fig. 1 , glucocorticoid therapy did not significantly influence the absorption of lung lesions. In addition, the median duration from treatment to residual lung lesions of glucocorticoid therapy patients was 26 (IQR, 22−27) days.
Fig. 1.
Chest CT results of glucocorticoid therapy COVID-19 pneumonia case and non-glucocorticoid therapy COVID-19 pneumonia case. (a) Representative chest CT results of glucocorticoid therapy COVID-19 pneumonia case, patient received glucocorticoid therapy on the 10th day after treatment; (b) representative chest CT results of non-glucocorticoid therapy COVID-19 pneumonia case. Both patients experienced a complete initial CT scan during the progressive and absorptive stage.
There was no significant difference with regard to the negative conversion rate of viral nucleic acid and duration time of negative transformation of viral nucleic acid between the glucocorticoid therapy and the non-glucocorticoid therapy groups among confirmed patients (median duration, 18 days [IQR, 15−23] vs 20 days [IQR, 18−23], p=0.55).
In our cohort of 104 patients with COVID-19 pneumonia, there were no major life-threatening adverse events. Among all patients, adverse effects attributable to drug treatment included infections (defined as hypokalaemia; total 23.1 %, glucocorticoid therapy group 24.4 %, non-glucocorticoid group 16.7 %; p = 0.69), secondary adverse reaction (defined as random blood glucose ≥11.1 mmol/L; total 20.2 %, glucocorticoid therapy group 22.1 %, non-glucocorticoid group 11.1 %; p = 0.46). There was no significant difference in the proportion of people with elevated blood glucose and hypokalaemia between the glucocorticoid therapy and non-glucocorticoid therapy groups.
As of March 14, 2020, among the glucocorticoid therapy group, 73 out of 86 patients were cured, 13 patients were still in hospital, and there were no deaths. Moreover, there was no statistical difference in the cure rate of COVID-19 pneumonia patients between the glucocorticoid therapy and non-glucocorticoid therapy groups (73 [84.9 %] vs 15 [83.3 %], p = 0.85).
4. Discussion
During the treatment of viral pneumonia, the effective glucocorticoid methylprednisolone sodium succinate is commonly used due to its quick action, short biological half-life and good safety, which is also the only hormonal drug that can be used for impact therapy. The anti-inflammatory activity of glucocorticoids is attributed to the repression of pro-inflammatory genes through signal transduction by their steroid receptor, the glucocorticoid receptor (GR), and GR signalling can play a dual role in the regulation of the immune response [11]. Glucocorticoids had been widely used in the treatment of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). However, use of glucocorticoids remains controversial for anti-inflammatory therapy of COVID-19 pneumonia [12,13]. The latest review article published in the journal Lancet reported that the clinical evidence of glucocorticoid on the treatment of COVID-19 pneumonia was limited and did not support the use of glucocorticoid [14]. Subsequently, a COVID-19 pneumonia study was published in JAMA [7], and it revealed that 45 % of the 128 patients were treated with steroids, but with no significant effect. However, related research [15,16] proved that low-dose glucocorticoids needed to be used as anti-inflammatory treatments as soon as possible, and reasonable use of glucocorticoid could reduce mortality. The results of a multicentre randomised controlled trial of dexamethasone in the treatment of acute respiratory distress syndrome (ARDS) published in Lancet [17] showed that early administration of dexamethasone could reduce mechanical ventilation time and overall mortality in patients with moderate to severe ARDS. A descriptive study of COVID-19 pneumonia [18] found that 19 % of patients received glucocorticoid therapy, and the recommended use of methylprednisolone could shorten the treatment time for severe mixed infection ARDS patients. On February 11, 2020, another newsletter was published in Lancet [19], which pointed out that inconclusive clinical evidence should not be a reason to abandon the use of glucocorticoids in COVID-19 pneumonia treatment.
The present study was a retrospective research to address glucocorticoid therapy for COVID-19 pneumonia patients. In the present study, patients with comorbidities and older age were more likely to receive glucocorticoid therapy, which was consistent with the clinician's criteria for using glucocorticoids. A previous study confirmed that increased age was associated with death in patients with COVID-19 pneumonia [18]. The patients receiving glucocorticoid therapy tended to have lower blood oxygen saturation and more severe lung imaging than their counterparts, which was consistent with the standard of clinicians who chose to use glucocorticoid therapy. The most common laboratory abnormalities observed of COVID-19 pneumonia patients were depressed total lymphocytes, prolonged prothrombin time, and elevated CRP. Similarly, lymphocyte count and CRP differ between glucocorticoid therapy and non-glucocorticoid therapy groups. Chinese management guidelines for COVID-19 pneumonia (version 5.0) issued by the National Health Commission of the People’s Republic of China added a new section of severe and critical clinical warning indicators, which aimed to alert clinicians to closely monitor indicators in clinical diagnosis and treatment, such as progressive decrease of peripheral lymphocytes and lactic acid. These changes could indicate the risk of progression to severe disease in patients [20]. In the present study, decrease of peripheral lymphocytes and the rapid increase of CRP in the glucocorticoid therapy group demonstrated that patients had poor basic condition and severe symptoms.
In the current study, we found that frequency of visit and duration of consultation
did not differ between the glucocorticoid therapy and non-glucocorticoid therapy groups, and it suggested that glucocorticoid therapy probably would not shorten the course of the disease. Moreover, the median duration of fever after treatment was 8 days. Imaging performance of COVID-19 pneumonia is divided into 4 stages: early stage, progressive stage, severe stage and absorptive stage. The CT imaging of the patients in the early stage of the disease showed that the density of the inflammatory focus of the lung was very low, which was more likely to be patchy or segmental than that of the localized lesion in the form of cloud and ground glass. As the course of the disease progresses, the focus of the disease increases, the lesion enlarges, and multiple lobes of the lung are involved, and the disease coexists with consolidation or streak shadow, If the viral pneumonia has not been effectively suppressed in the clinic, it will enter the severe stage [21,22]. The chest imaging manifestations of COVID-19 pneumonia patients can appear in 4 stages in sequence, or they may jump. For example, early lesions can directly enter the absorptive stage without going through the progressive stage or severe stage. However, because of the longer interval of chest CT review, the patient may miss the imaging progressive stage and directly enter the absorptive stage during the consultation. Therefore, it is more accurate to observe the clinical efficacy of patients with complete imaging in the progressive and absorption stages, which is one reason why the clinical course analysis of this study included only 104 cases. The time from the first CT scan to the first discovery of radiographic progression was 4 (IQR,3−5) days, which suggested that the patients should receive re-examination of chest CT in a short time to ensure timely treatment and intervention. We found that glucocorticoid therapy did not affect the imaging process in this study. Studies had shown that when SARS patients had increased lung shadow and dyspnoea, early and appropriate use of glucocorticoids could significantly improve the clinical symptoms of patients, reduce the degree of disease progression and accelerate the absorption of lung lesions. The data in this study showed that recovery of the patient's lung was a gradual absorptive process, with a median time of 11 days. For general COVID-19 pneumonia patients, clinicians should be careful when choosing glucocorticoid therapy, such as persistent high fever, obvious dyspnoea, obvious progress in imaging and other inflammatory manifestations, and appropriate use of glucocorticoids is reasonable.
Patients began glucocorticoid therapy in the first 7 (IQR,6−10) days after onset of illness. Nucleic acid of all patients had eventually turned negative during the follow-up period. Our results revealed that glucocorticoid therapy was not significantly associated with delay in COVID-19 pneumonia negative transformation of nucleic acid. After adjustment for baseline and time-varying confounders, the use of corticosteroid therapy was not associated with 90-day mortality but was associated with delayed MERS-CoV RNA clearance [23].
There were many adverse events of glucocorticoids in the treatment of other viral pneumonia. The glucocorticoid treatment of SARS patients in 2003, due to the use of dose, starting time and course of treatment was not very effective, resulting in some patients with serious complications [24], such as invasive fungal infection and osteonecrosis of the femoral head. Related studies showed that high doses of glucocorticoids can significantly increase the incidence of diabetes in patients. Our study found that there were no significant association between glucocorticoid therapy and adverse events, which may be related to the small dose of glucocorticoid we use.
Our study has some limitations. First, our findings might be limited by the small sample size, and a larger cohort study was needed to verify our conclusions. Second, due to the retrospective study design, the clinical characteristics of the two groups of patients could not be matched exactly, and no immunological testing was performed to understand the potential effect of glucocorticoids on dumping the immune response. Third, among the 104 cases, some patients were still hospitalized at the time of manuscript submission, therefore, it was difficult to assess the prognostic risk factors, and continued observations of the disease were needed. Fourth, there was no multivariate analysis. Finally, not all patients had complete treatment data, and follow-up information interpretation of our findings might be limited by the sample size.
5. Conclusion
The present study mainly focused on the role of low-dose glucocorticoid therapy on COVID-19 pneumonia patients. Our retrospective study indicated that glucocorticoid therapy did not significantly influence the clinical course, adverse events nor the outcome of COVID-19 pneumonia. For the treatment of COVID-19 pneumonia, the timing and dosage of glucocorticoids still requires in-depth and systematic investigation to ensure that it inhibits overwhelming inflammatory response rather than the protective immune response to COVID-19 pneumonia.
Funding source
None.
Declaration of Competing Interest
There are no conflicts to declare.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
References
- 1.Panahi L., Amiri M., Pouy S. Risks of novel coronavirus disease (COVID-19) in pregnancy; a narrative review. Archi. Acad. Emerg. Med. 2020;8:e34. [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu H., Wei L., Niu P. The novel coronavirus outbreak in Wuhan, China. Glob. Health Res. Policy. 2020;5:6. doi: 10.1186/s41256-020-00135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phelan A.L., Katz R., Gostin L.O. The novel coronavirus originating in Wuhan, China: challenges for global health governance. Jama. 2020 doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- 5.National Health Commission of the People’s Republic of China; 2020. National Health Commission of the People’s Republic of China, Chinese Management Guideline for COVID-19 (version 5.0)http://www.nhc.gov.cn/yzygj/s7653p/202002/d4b895337e19445f8d728fcaf1e3e13a/files/ab6bec7f93e64e7f998d802991203cd6.pdf https://doi:www.nhc.gov.cn/yzygj/s7653p/202002/d4b895337e19445f8d728fcaf1e3e13a/files/ab6bec7f93e64e7f998d802991203cd6.pdf. [Google Scholar]
- 6.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. Jama. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garfin D.R., Silver R.C., Holman E.A. The novel coronavirus (COVID-2019) outbreak: amplification of public health consequences by media exposure. Health Psychol. 2020;39:355–357. doi: 10.1037/hea0000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lupia T., Scabini S., Mornese Pinna S., Di Perri G., De Rosa F.G., Corcione S. Novel coronavirus (2019-nCoV) outbreak: a new challenge. J. Glob. Antimicrob. Resist. 2019;21(2020):22–27. doi: 10.1016/j.jgar.2020.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X., Liu W., Zhao J., Lu Y., Wang X., Yu C., Hu S., Shen N., Liu W., Sun Z., Li W. Clinical characteristics of 80 hospitalized frontline medical workers infected with COVID-19 in Wuhan, China. J. Hosp. Infect. 2020;105:399–403. doi: 10.1016/j.jhin.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz-Topete D., Cidlowski J.A. One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation. 2015;22:20–32. doi: 10.1159/000362724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J., Zheng X., Tong Q., Li W., Wang B., Sutter K., Trilling M., Lu M., Dittmer U., Yang D. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J. Med. Virol. 2020;92:491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H., Li J., Chen M., Su J. Glucocorticoid treatment of suspected organizing pneumonia after H7N9 infection: a case report. Medicine. 2019;98:e16839. doi: 10.1097/MD.0000000000016839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Z., Zhang F., Xu M., Huang K., Zhong W., Cai W., Yin Z., Huang S., Deng Z., Wei M., Xiong J., Hawkey P.M. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. J. Med. Microbiol. 2003;52:715–720. doi: 10.1099/jmm.0.05320-0. [DOI] [PubMed] [Google Scholar]
- 16.Chen R.C., Tang X.P., Tan S.Y., Liang B.L., Wan Z.Y., Fang J.Q., Zhong N. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129:1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villar J., Ferrando C., Martinez D., Ambros A., Munoz T., Soler J.A., Aguilar G., Alba F., Gonzalez-Higueras E., Conesa L.A., Martin-Rodriguez C., Diaz-Dominguez F.J., Serna-Grande P., Rivas R., Ferreres J., Belda J., Capilla L., Tallet A., Anon J.M., Fernandez R.L., Gonzalez-Martin J.M., Ards network dexamethasone in Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir. Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 18.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie J., Tong Z., Guan X., Du B., Qiu H., Slutsky A.S. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. 2020;46:837–840. doi: 10.1007/s00134-020-05979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X., Song W., Liu X., Lyu L. CT image of novel coronavirus pneumonia: a case report. J. Radiol. 2020;38:407–408. doi: 10.1007/s11604-020-00945-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y., Liu Y.L., Li Z.P., Kuang J.Y., Li X.M., Yang Y.Y., Feng S.T. Clinical and CT imaging features of 2019 novel coronavirus disease (COVID-19) J. Infect. 2020;81:147–178. doi: 10.1016/j.jinf.2020.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A., Jose J., Pinto R., Al-Omari A., Kharaba A., Almotairi A., Al Khatib K., Alraddadi B., Shalhoub S., Abdulmomen A., Qushmaq I., Mady A., Solaiman O., Al-Aithan A.M., Al-Raddadi R., Ragab A., Balkhy H.H., Al Harthy A., Deeb A.M., Al Mutairi H., Al-Dawood A., Merson L., Hayden F.G., Fowler R.A., Group Saudi Critical Care Trial Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am. J. Respir. Crit. Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 24.Auyeung T.W., Lee J.S., Lai W.K., Choi C.H., Lee H.K., Lee J.S., Li P.C., Lok K.H., Ng Y.Y., Wong W.M., Yeung Y.M. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J. Infect. 2005;51:98–102. doi: 10.1016/j.jinf.2004.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]