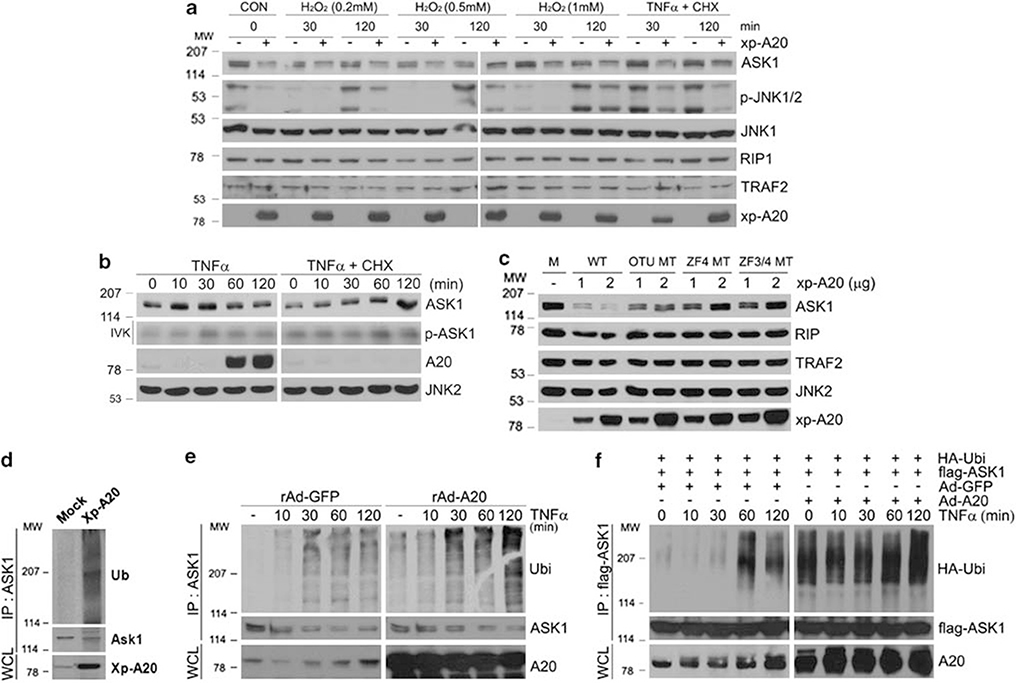
Figure 7.
A20 reduces the stability and promotes the degradation of ASK1 through the ubiquitination process. (a) Control empty vector or Xp-tagged A20 constructs were transfected into HEK293 cells, followed by treatment with various concentrations of H2O2 or TNF (15 ng/ml) plus CHX (10 μg/ml) for indicated times. Whole cell extracts were subjected to immunoblotting with anti-ASK1, phospho-JNK, JNK1, RIP1, and TRAF2 antibodies. Expression levels of the transfected A20 were analyzed by immunoblotting with anti-Xp antibody. (b) HeLa cells were treated with either TNF (15 ng/ml) or TNF (15 ng/ml) plus CHX (10 μg/ml) for indicated times. Whole cell extracts were immunoprecipitated with anti-ASK1 antibody and autophosphorylation of ASK1 was examined by immune complex kinase assay (second row). The same extracts were subjected to immunoblotting with anti-ASK1, A20, and JNK antibodies (top and bottom two rows). (c) HEK293 cells were transfected with indicated plasmids for 30 h, and whole cell extracts were subjected to immunoblotting with anti-ASK1, RIP1, TRAF2, and JNK antibodies. Expression levels of the transfected proteins were analyzed as in (a). (d) HEK293 cells were transfected with control empty vector or Xp-tagged A20 for 30 h. Cells were lysed and ASK1 was immunoprecipitated with anti-ASK1 followed by immunoblotting with anti-Ub antibody (top). (e) HEK293 cells were infected with either rAd-GFP or rAd-A20 as described in Figure 1a, and the cells were treated with TNF for the indicated times. Cells were lysed and ASK1 ubiquitination was examined as described in (d). (f) HEK293 cells expressing with either rAd-GFP or rAd-A20 were transfected with flag-ASK1 and HA-Ub. After 30 h, the cells were treated with TNF for the indicated times and immunoprecipitated with anti-flag antibody followed by immunoblotting with anti-HA antibody (top)