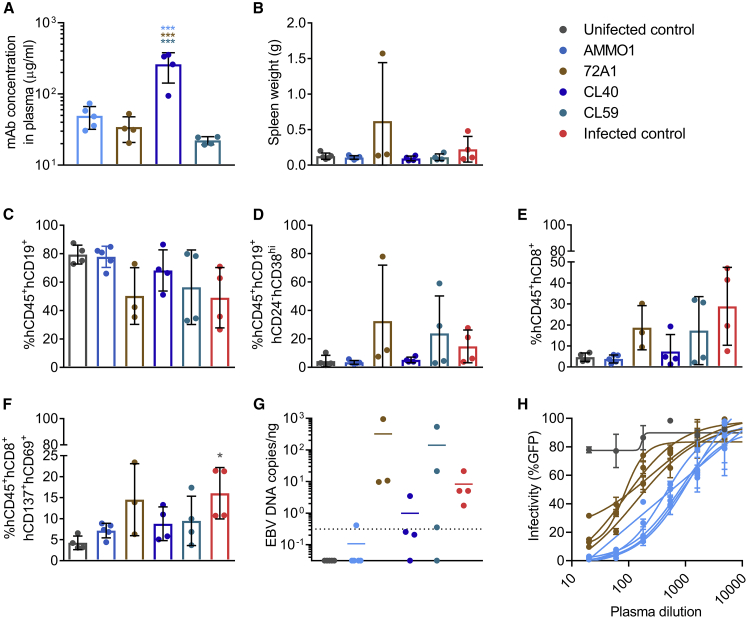
Figure 2.
AMMO1 Inhibits EBV Infection More Efficiently Than Other Anti-EBV mAbs in Humanized Mice
Humanized mice received an i.v. injection containing 0.5 mg of the mAbs AMMO1 (n = 5), 72A1 (n = 4), CL40 (n = 4), and CL59 (n = 4). Two days later, the mice were challenged with 33,000 Raji infectious units of EBV B95.8/F.
(A) One week following challenge, the concentration of transferred mAbs in plasma was determined by antigen-specific ELISA against gH/gL for AMMO1, CL40 and CL59, and gp350 for 72A1.
(B) Spleen weight of study animals 8 weeks after challenge. Uninfected control mice (n = 4) that did not receive antibody or virus and infected control mice (n = 4) that were challenged with the virus but did not receive antibody pre-treatment are included.
(C–F) At necropsy, the frequency of (C) hCD45+hCD19+, (D) hCD45+hCD19+hCD24−hCD38hi, (E) hCD45+hCD8+, and (F) hCD8+hCD69+hCD137+ cells were measured in splenocytes.
(G) qPCR was used to quantitate viral DNA in splenic extracts at necropsy. Each data point represents an individual mouse, the bar indicates the mean, and the dashed line indicates the limit of detection.
(H) The EBV-neutralizing titers in plasma from AMMO1- or 72A1-infused mice were measured 1 week following challenge.
Bar graphs indicate the mean, error bars represent the standard deviation, and each data point represents an individual mouse in (A)–(F). One of the animals in the 72A1 group died prior to necropsy; therefore, only 3 mice are shown in (B)–(G). Statistical analyses were performed using one-way ANOVA. The color of the asterisks (*p ≤ 0.0332, ***p ≤ 0.0002) denotes the group with which there is a significant difference, determined by a Sidak multiple comparisons test.