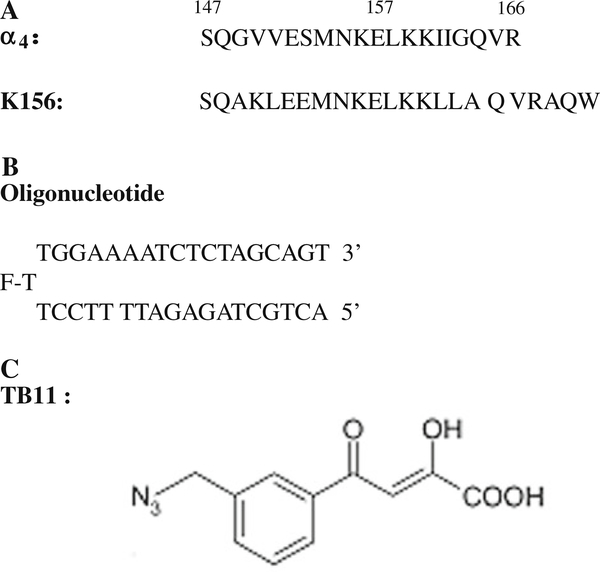
Fig. 2.
The compounds under studies: a Sequences of the α4 peptide and of its structural analog K156; b The oligonucleotide is designed to adopt a hairpin structure with a three thymine (T) loop (the central thymine bears the fluorophore (fluorescein) and a 17-base pair stem). The latter reproduces the end of the U5ĽTR of HIV-1 DNA but plays the role of both virus DNA and cell DNA in in vitro experiments; c The IN inhibitor TB11 includes the critical DKA group and also an aryl ring with an azide substitutent important for biological activity [3, 19]