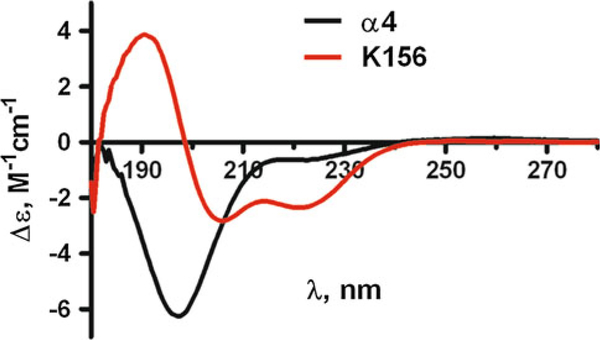
Fig. 3.
Circular dichroism spectra of the α4 and K156 peptides in buffer (see "Experimental Procedure") at 10 C in the far UV region (180–250 nm). The α4 peptide spectrum with its deep negative band at ≈195 nm is characteristic of random coil structures. The K156 spectrum is mostly of helix type with its two negative bands at ≈222 nm and ≈208 nm and its positive band at ≈190 nm