Figure 2.
Overall Structure of the SARS-CoV2 nsp13 Helicase with the Holo-RdRp:RNA Replication-Transcription Complex (RTC)
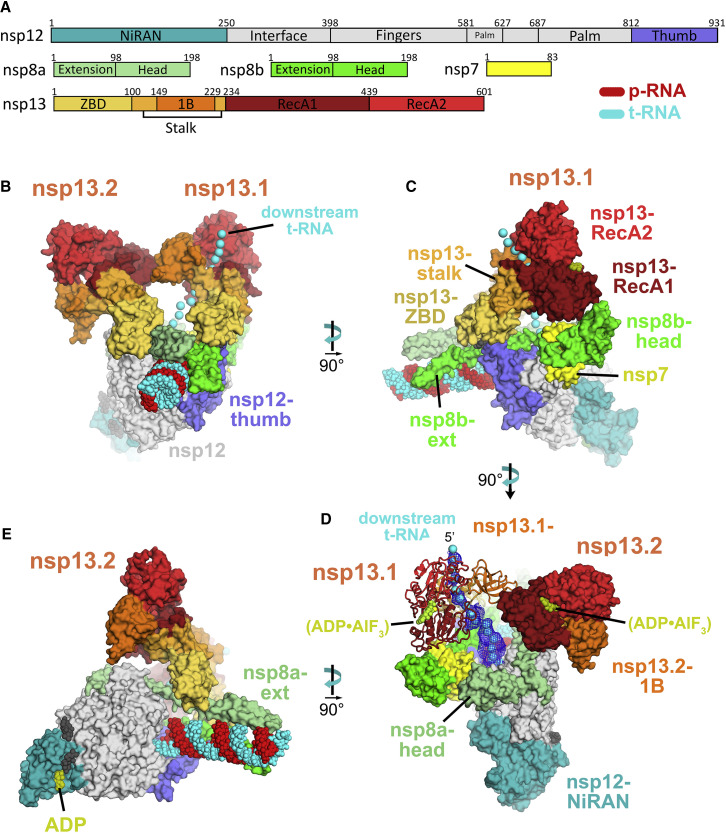
(A) Schematic illustrating the domain structure of SARS-CoV-2 holo-RdRp (nsp7, nsp8, and nsp12) and nsp13. Structural domains discussed in the text are labeled. The color coding corresponds to the figures throughout this manuscript unless otherwise specified.
(B–E) Orthogonal views showing the overall architecture of the nsp132-RTC. Proteins are shown as molecular surfaces (except nsp13.1 in D) and RNA as atomic spheres. Adventitious CHAPSO detergent molecules are shown as atomic spheres and colored dark gray. The path of downstream tRNA through the nsp13.1 helicase, shown as cyan spheres, is revealed with low-pass-filtered (6 Å) difference density (shown in D).
(B) Two copies of the nsp13 helicase bind to the RTC. Nsp13.1 forms a tripartite interaction with the nsp8b extension and the nsp12 thumb via the nsp13.1-ZBD. The 5′ end of the tRNA extrudes through the nucleic acid binding channel of nsp13.1. The two helicases interact via the nsp13.1-1B domain and the nsp13.2-RecA1 domain.
(C) In addition to the nsp13.1-ZBD:nsp8b extension:nsp12 thumb tripartite interaction, nsp13.1-RecA1 interacts with nsp7 and the nsp8b head.
(D) ADP-AlF3 is modeled in the NTP binding site of each helicase. The low-pass-filtered (6 Å) cryo-EM difference density revealing the path of the downstream t-RNA is shown (dark blue mesh).
(E) The nsp13.2-ZBD interacts with the nsp8a extension. ADP-Mg2+ is bound to the NiRAN domain.
See also Figure S3, Figure S4, Figure S5, Figure S6, Table S1, and Video S1.