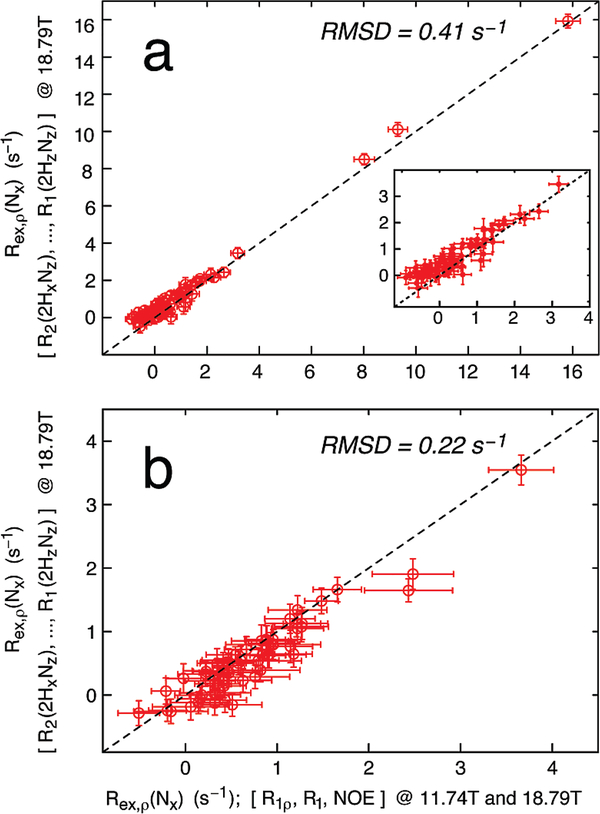
Figure 1.
Exchange contributions Rex,ρ(Nx) obtained from measurement of residue-specific R2(2HxNz), R2(2HzNx), R2(2HxNx), and R1(2HzNz) relaxation rates at a magnetic field strength of 18.8 T (y-axis) agree with the corresponding values isolated from 15N R1, 15N R1ρ, and 1H–15N NOE measurements at two static magnetic fields (11.7 and 18.8 T) for U-2H,15N-labeled human ubiquitin (a) and U-2H,15N-labeled protein L (b), 278 K. A 15N spin-lock field strength of 2 kHz was used for all experiments. The dashed lines correspond to y = x, and the rmsd is calculated as . The inset in (a) shows an expansion of the region corresponding to small Rex,ρ(Nx) rates. A similar plot is shown in the Supporting Information where Rex,ρ(Nx) was obtained from R2(2HxNz), R2(2HzNx), R2(2HxNx), and R1(2HzNz) relaxation rates measured at a magnetic field strength of 11.7 T.