Figure 1:
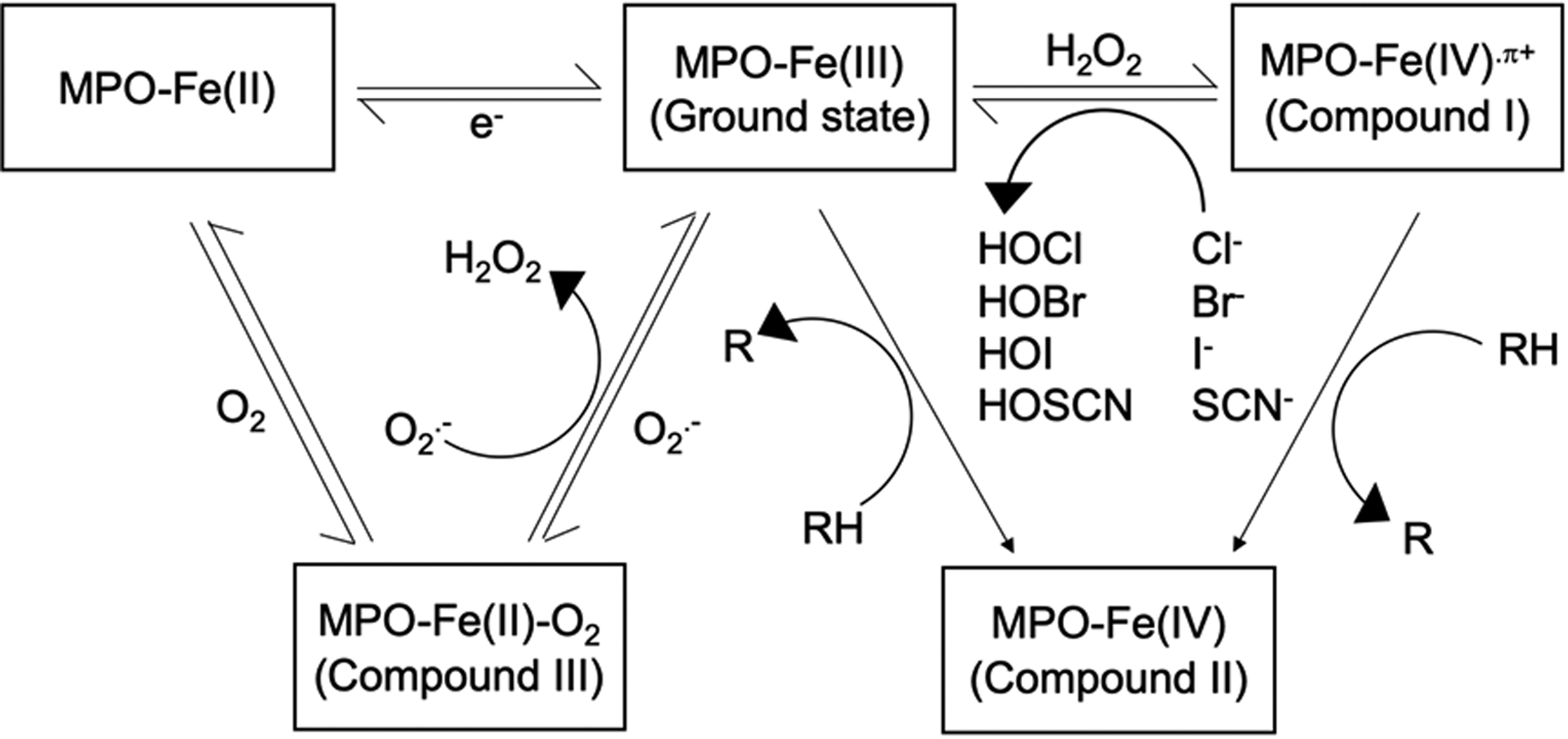
The catalytic cycle of myeloperoxidase. Ground state MPO (MPO-Fe(III)) is oxidized by H2O2 to become Compound I (MPO-Fe(IV).π+), which can be reduced to the ground state MPO by either halogenation cycle, or peroxidation cycle, depending on the concentration of H2O2. In halogenation cycle, halide (Cl−, Br−, or I−) or pseudohalide (SCN−) is used to produce hypohalous acid (HOCl, HOBr, HOI, or HOSCN). In peroxidation cycle, Compound II (MPO-Fe(IV)) is formed as an intermediate. Ground state MPO can also be reduced to MPO-Fe(II) or Compound III (MPO-Fe(II)-O2) by e− or O2.-, respectively. MPO-Fe(II) can be transformed back to the ground state form, or to Compound III by binding to O2. Compound III can also be converted back to the ground state form by O2.-, resulting in the formation of H2O2.
