Figure 3.
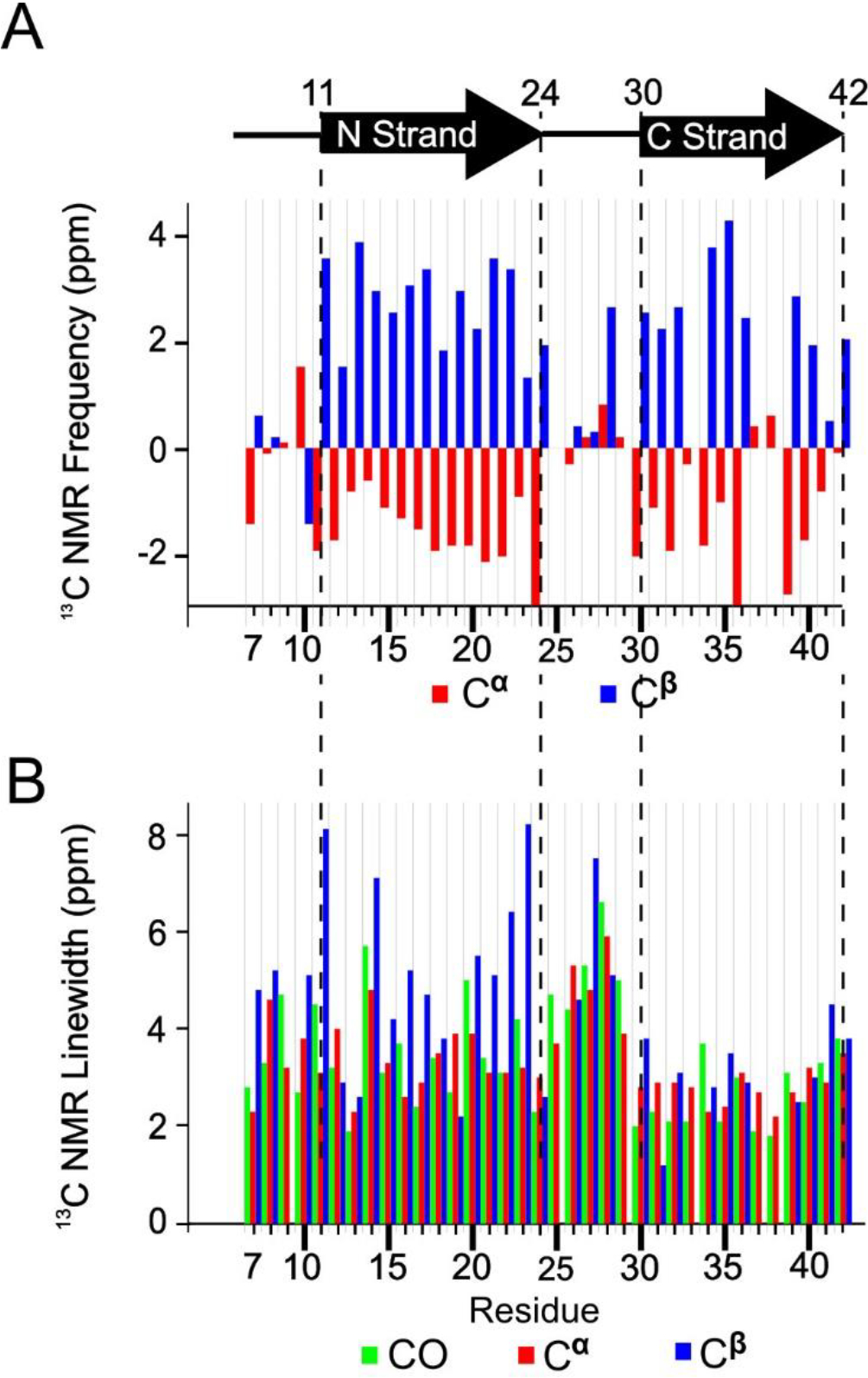
A) Secondary structure of peptide residues in the 150 kDa Aβ(1–42) oligomer, predicted by TALOS-N software, and the measured secondary 13C NMR backbone chemical shifts upon which the TALOS-N prediction is based. B) The NMR linewidths of CO (green), Cα (red), and Cβ (blue) reported as full widths at half maximum measured by nonlinear regression of cross peaks in the 2D-fpRFDR 13C-13C NMR spectra to Gaussian functions.
