Abstract
Novel antimicrobials are urgently needed to combat drug-resistant bacteria and to overcome the inherent difficulties in treating biofilm-associated infections. Studying plants and other natural materials used in historical infection remedies may enable further discoveries to help fill the antibiotic discovery gap. We previously reconstructed a 1,000-year-old remedy containing onion, garlic, wine, and bile salts, known as ‘Bald’s eyesalve’, and showed it had promising antibacterial activity. In this current paper, we have found this bactericidal activity extends to a range of Gram-negative and Gram-positive wound pathogens in planktonic culture and, crucially, that this activity is maintained against Acinetobacter baumannii, Stenotrophomonas maltophilia, Staphylococcus aureus, Staphylococcus epidermidis and Streptococcus pyogenes in a soft-tissue wound biofilm model. While the presence of garlic in the mixture can explain the activity against planktonic cultures, garlic has no activity against biofilms. We have found the potent anti-biofilm activity of Bald’s eyesalve cannot be attributed to a single ingredient and requires the combination of all ingredients to achieve full activity. Our work highlights the need to explore not only single compounds but also mixtures of natural products for treating biofilm infections and underlines the importance of working with biofilm models when exploring natural products for the anti-biofilm pipeline.
Subject terms: Antibiotics, Biofilms, Drug discovery
Introduction
Widespread multidrug resistance of once-susceptible pathogens, combined with a lack of success in developing novel antimicrobials, has resulted in a looming crisis. Antimicrobial resistance leads to problematic infections, threatens the success of routine surgery and cancer treatments1, and is estimated to kill 10 million people per year by 20502,3. One particularly troublesome area is biofilm-associated infection. Biofilm infections are estimated to cost the UK’s National Health Service over a billion pounds every year, with this cost only set to increase4,5. Biofilms are communities of bacteria that produce a protective extracellular matrix and are especially persistent6. Biofilm eradication often requires 100–1,000 times higher antibiotic concentrations to achieve clearance than the same bacteria growing planktonically (as individual free-floating cells)7. In vivo, biofilms may essentially be completely impervious to antibiotic treatment8.
The path to overcoming biofilm infections requires a multifaceted response, including the discovery and clinical deployment of novel antimicrobials. This search for novel candidates must be directed towards those pathogenic bacteria with the highest health and economic impact (e.g. ESKAPE group9,10) and will be expedited if early discovery work on leads takes into account the inherent difficulties of treating biofilm-associated infections6,11.
The heightened urgency for novel antibiotics has resulted in natural products being revisited. Just 200 years ago, our pharmacopoeia was dominated by herbal medicines. These medicines have had varying levels of success for the treatment of infections in modern research, with a number of potent compounds against planktonic cultures being identified12,13; however, these have been far less successful against biofilm cultures. We do not fully understand the reasons for this lack of translational success of plant extracts.
It could be that the conventional process for developing drugs may miss key aspects of those herbal remedies which could be effective against biofilms. Conventional drug development calls for the isolation of single active compounds, whereas historical medicine usually calls for combinations of whole plants (and other natural materials). There is some evidence that whole plant extracts can have stronger biological effects than individual isolated compounds14. Examples of such synergy include observations that the anti-malarial activity of artemisinin is enhanced by the presence of other compounds from the same plant15,16 or the combination of flavonoids used in Citrox14. Synergy may result from the presence of molecules which potentiate the activity of antimicrobial compounds, or from multiple active compounds with different mechanisms of action. It is also possible that in purifying individual compounds to achieve readily quantifiable and characterised treatments, we may lose vital interactions of natural products within the original mixture which prevent irritation or toxicity.
Additionally, research into natural antimicrobial products is often limited by a methodological focus on planktonic activity testing. The current gold standard for antibacterial testing is broth microdilution, usually conducted in cation-adjusted Mueller–Hinton Broth17, and this is used extensively for the discovery of novel antibacterial compounds. However, whilst providing a great high-throughput screening method, this does not provide information on how the test substance interacts with bacteria in biofilms, or in settings which better mimic the chemical environment experienced by pathogens in vivo17,18. This focus may in part explain the lack of translational success of isolated plant compounds, as their activity may be sufficient to kill planktonic cells, but not to penetrate or kill biofilms—i.e. by not using biofilm assays, we create a high false positive rate in the early stages of activity testing.
In this paper, we investigate the importance of the combination of ingredients for the anti-biofilm activity of a tenth century remedy used for eye infection from the manuscript known as Bald’s Leechbook (London, British Library, Royal 12, D xvii). This remedy has previously been shown to kill S. aureus biofilms by Harrison et al. (2015)19, Pseudomonas aeruginosa planktonic cultures20 and recently Neisseria gonorrhoeae in a disk diffusion assay21. The recipe, known as ‘Bald’s eyesalve’, requires equal volumes of garlic (Allium sativum) and another Allium species (referred to as cropleac in the original Old English) to be crushed together and mixed with equal volumes of wine and ox gall (bovine bile). We now report two key new findings. First, we show the bactericidal activity of Bald’s eyesalve against a panel of clinically relevant strains of ESKAPE pathogens in planktonic culture, and against biofilms of a subset of these strains. Second, we conducted a detailed assessment of whether (a) any individual ingredient or (b) the sulphur-containing compound allicin (from garlic) can explain this activity. No single ingredient recaptures the anti-biofilm activity of Bald’s eyesalve, and the presence of allicin cannot fully explain the ability of the whole mixture to kill either planktonic cultures grown in host-mimicking medium (synthetic wound fluid), or biofilms grown in an in vivo-like model of a soft tissue wound. The combination of ingredients in the full mixture is the key to the remedy’s apparent efficacy, and this is only apparent when it is tested in host-mimicking models as opposed to planktonic cultures in standard laboratory medium.
Results
Consistent anti-biofilm activity of Bald’s eyesalve and decision to use onion (Allium cepa) as “cropleac”
The meaning of Old English cropleac, as used in the original remedy, is ambiguous and may refer to a variety of Allium species22,23. Two likely translations are onion (Allium cepa) or leek (Allium porrum). For this reason, multiple batches of the remedy using either Allium species were made in our laboratory between 2014 and 2019. After the 9-day brewing period, the activity of the remedies was tested against mature biofilms of S. aureus Newman in an in vivo-like model of a soft tissue wound. In this assay, 24-h-old biofilms, that have been grown at 37 °C in collagen-based synthetic wounds, are exposed to Bald’s eyesalve or water for 24 h, and surviving bacteria are counted19,24.
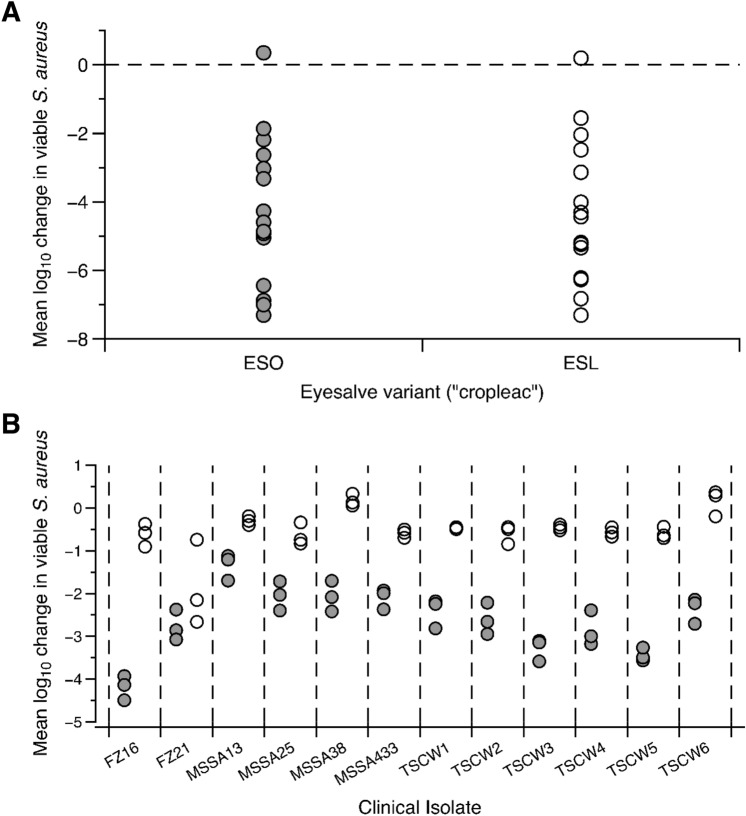
In total, 75 batches of Bald’s eyesalve were made, including 15 pairs of batches where onion (ESO) and leek (ESL) variants were made at the same time, using the same garlic, wine and bile. As shown in Fig. 1A, biofilm killing was achieved by 14/15 paired batches, with 22/30 preparations causing a > 3-log drop in viable bacteria, when compared with water-treated control biofilms. The mean log drop was not significantly different between paired ESO and ESL batches (paired t-test t14 = 0.025, p = 0.981). We tested one example pair of ESO and ESL variants (batch 6) against a panel of twelve clinical isolates of S. aureus grown in the synthetic wound biofilm model. As shown in Fig. 1B, ESO consistently showed more bactericidal activity than ESL. This result may be due to the presence of antimicrobial compounds unique to onion, or it may simply be due to onions being easier to crush in a mortar and pestle, potentially making the extraction of natural compounds more efficient. To simplify further analysis, follow on work was focussed on ESO.
Figure 1.
The activity of Bald’s eyesalve, ESO and ESL variants, against S. aureus. (A) 15 pairs of Bald’s eyesalve translating cropleac as either onion (ESO, grey) and leek (ESL, white), were prepared at the same time (batches 1–15). Their activity was assessed against S. aureus Newman biofilms. Mature biofilms of S. aureus Newman were grown in a model of a soft tissue wound, then treated with either sterile water or Bald’s eyesalve (n = 3–5 replicates per treatment) for 24 h before recovering bacteria for CFU counts. The mean log change in viable bacteria in treated vs control wounds was calculated for each preparation and no significant difference was seen (paired t-test t14 = 0.025, p = 0.981). Raw data are supplied in the Data Supplement. (B) Six carriage isolates of S. aureus, and six isolates from a chronic post-surgical wound (detailed in Table 4), were grown in the synthetic wound biofilm model and treated with ESO, ESL or water (n = 3 per treatment). Each strain grew to different densities in untreated wounds (range approx. 106–108). The log10 drop in bacteria associated with treatment was calculated for each treated wound relative to the mean CFU in the three untreated wounds. Log drop data was analysed by ANOVA which revealed significant differences in log drop between eyesalve variants (F1,48 = 732, p < 0.001) and strains (F11,48 = 14.4, p < 0.001) and a significant strain-dependent difference in the magnitude of the effect of eyesalve variant (strain*variant interaction F11,48 = 8.66, p < 0.001). The family-wise error rate was calculated from the ANOVA table and used to conduct planned contrasts of ESO vs ESL treatment effects for each strain using t-tests. ESO caused a larger reduction in viable cells than ESL for all strains (all p < 0.001). Raw data and R scripts are supplied in the Data Supplement.
As shown in Fig. 2, biofilm killing was consistently achieved by multiple batches of ESO. Of these batches, 62 of the 75 caused a > 3-log drop in viable CFU, with 45/75 causing > 5 log drop, and 28 of these causing > 6-log drop, all relative to control biofilms treated with sterile water.
Figure 2.
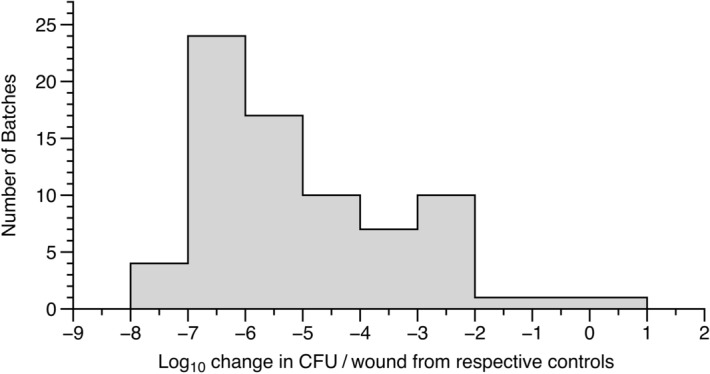
The activity of 75 Bald’s eyesalve (ESO) batches made by our group against S. aureus Newman biofilms. Mature S. aureus Newman biofilms were grown in a model of a soft tissue wound, then treated with either sterile water (control) or eyesalve (n = 3–5 replicates per treatment) for 24 h before recovering bacteria for CFU counts. All batches showed killing, and the log drop in treated vs control wounds was calculated. Raw data are provided in the Data Supplement.
Broad-spectrum antibacterial activity of Bald’s eyesalve against common wound pathogens
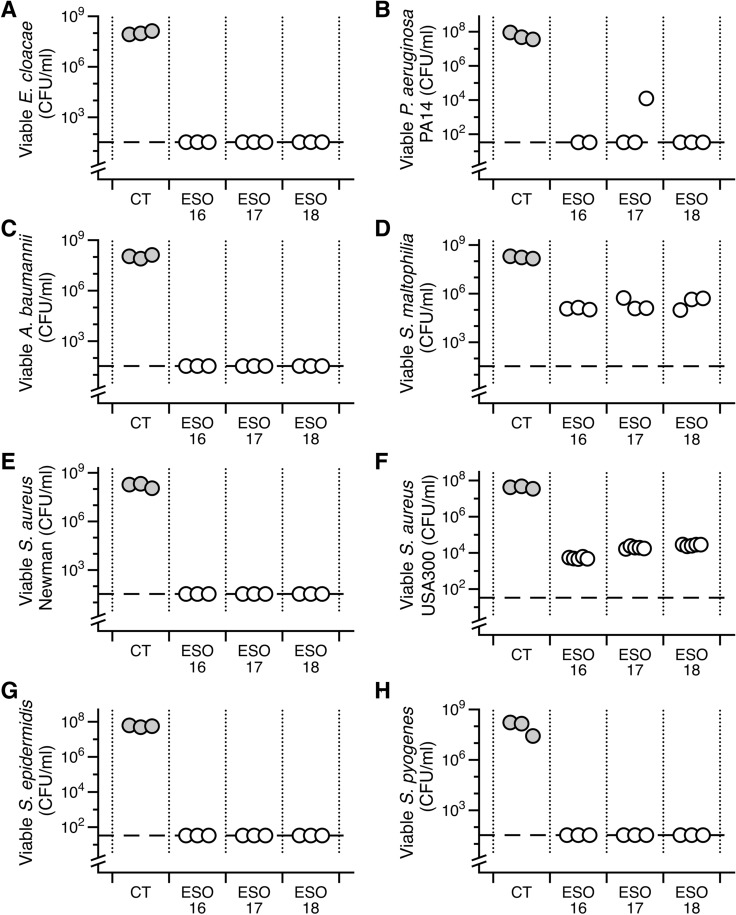
The antibacterial activity of three batches of ESO was tested in both planktonic and biofilm cultures of eight strains of bacteria that commonly cause chronic wound infections. As shown in Fig. 3, ESO had potent activity against planktonic cultures of Gram-negative (P. aeruginosa PA14, A. baumannii clinical isolate, E. cloacae, S. maltophilia) and Gram-positive (S. aureus Newman, S. aureus USA300, S. epidermidis and S. pyogenes) wound pathogens. ESO eradicated all planktonic cultures in all strains tested with the exception of S. aureus USA300 and S. maltophilia, where a 3–4 log drop in viable bacteria was seen.
Figure 3.
Bald’s eyesalve (ESO) activity against eight example strains of bacteria that commonly cause chronic wound infections. Planktonic cultures were grown for 6 h in synthetic wound fluid. Cultures were treated with ESO (batches 16–18) or sterile water (CT) to a final concentration of 33% v/v (n = 3–5 replicates per treatment). After 18 h bacteria were recovered for CFU counts. The dashed line represents the limit of detection by plating. For S. maltophila and S. aureus USA300, where we did not observe complete killing, we used ANOVA to determine that the CFU recovered from ESO-treated wounds was significantly different from control wounds (S. aureus USA300: F2,14 = 3,458, p < 0.001; S. maltophilia: F3,8 = 87.41, p < 0.001). Data were log transformed to meet the assumptions of linear modelling. A Dunnett’s test was conducted to compare the CFU of the three batches with the CFU of the controls. All 3 batches of ESO were significantly different from their respective controls for both isolates (all p < 0.001). Raw data and R scripts are supplied in the Data Supplement.
Mature biofilms of the above isolates were grown in the synthetic wound model and treated with the same batches of eyesalve used for planktonic killing experiments. A 2–6-log drop in viable cells was observed for the Gram-positives S. aureus Newman, S. aureus USA300, S. epidermidis, S. pyogenes and the Gram-negative A. baumannii (Fig. 4). No or inconsistent killing was observed for P. aeruginosa, E. cloacae and S. maltophilia biofilms (Fig. S3).
Figure 4.
Bald’s eyesalve (ESO) anti-biofilm activity. Mature biofilms of various isolates were grown in a model of a soft tissue wound, then treated with either sterile water (control, CT) or eyesalve (batches 16–18), to a final concentration of 33% (v/v), for 24 h before recovering bacteria for CFU counts (n = 3–5 replicates per treatment). The dashed line represents the limit of detection by plating. For S. aureus Newman, S. aureus USA300, and S. epidermidis, where we did not observe complete killing, we used ANOVA to determine that the CFU recovered from ESO-treated wounds was significantly different from control wounds (S. aureus Newman: F3,8 = 107, p < 0.001; S. aureus USA300: F3,14 = 103.5, p < 0.001; S. epidermidis: F3,14 = 63.86, p < 0.001). Data were log transformed to meet the assumptions of linear modelling. A Dunnett’s test was conducted to compare the CFU of the three batches with the CFU of the controls. All 3 batches of ESO were significantly different from their respective controls for all three isolates (all p < 0.001). Raw data and R scripts are supplied in the Data Supplement.
Garlic is responsible for the majority of planktonic killing by ESO
Our initial work indicated that all four ingredients in ESO were required to kill biofilms of S. aureus Newman in synthetic wound biofilms19. A recent publication by Fuchs et al. (2018;20) concluded that the bactericidal activity of ESO was due to the presence of allicin from garlic, however, the Fuchs et al. study only investigated planktonic killing in standard Mueller–Hinton Broth (MHB). It is well known that planktonic cultures of bacteria can be up to 1,000 times more sensitive to antibiotics than the same isolates grown as biofilms7, and that antibiotic sensitivity is highly dependent on growth medium25. This means conducting assays only on planktonic MHB cultures risks underestimating the number or concentration of bioactive agents in ESO required for killing in in vivo-like conditions17,18.
To more robustly determine if the activity of ESO stems from one ingredient or several, we prepared individual ingredients and preparations omitting one ingredient, such that the concentrations of each ingredient in the whole remedy and the single ingredient or dropout variants were equal. The MICs of these preparations, along with MICs of the full recipe, were assessed in MHB and in SWF, in standard broth microdilution assays using four of the isolates previously tested (two Gram-negatives, A. baumannii clinical isolate strain and P. aeruginosa PA14, and two Gram-positives, S. aureus Newman and S. aureus USA300).
As shown in Table 1, the bacterial isolates varied in their sensitivity to the full ESO. Crucially, there was an effect of the growth medium, such that MICs at least doubled in SWF compared with MHB for S. aureus Newman and A. baumannii. Wine, bile or onion alone were much less effective than the full remedy. In MHB, the MIC of garlic alone was the same as the ESO MIC for all strains except P. aeruginosa, and in SWF the garlic and ESO MICs were either equal or exhibited a max. twofold difference. This suggests that the planktonic activity of the remedy is due to the presence of garlic. The results of MIC testing with ingredients omitted gave similar results (Table 2). Preparations omitting garlic lost all or most of their antibacterial activity. Removal of any other ingredient left activity largely unaffected, although the omission of wine or bile doubled the MIC against P. aeruginosa PA14 in SWF. The discordance between MICs in different media is more prominent here, with the removal of an ingredient having a minimal effect in MHB but a large loss in activity when tested in SWF. This stresses the common discordance between testing in standard rich lab medium and host-mimicking medium.
Table 1.
Minimum inhibitory concentration (MIC) of ESO or individual ingredients.
| Treatment |
S. aureus Newman |
S. aureus USA300 |
A. baumannii clinical isolate |
P. aeruginosa PA14 |
||||
|---|---|---|---|---|---|---|---|---|
| MHB | SWF | MHB | SWF | MHB | SWF | MHB | SWF | |
| ESO | 1.56 | 6.25 | 3.13 | 3.13 | 1.56 | 3.13 | 25 | 25 |
| Onion | > 50 | > 50 | > 50 | > 50 | > 50 | > 50 | > 50 | > 50 |
| Garlic | 1.56 | 12.5 | 3.13 | 3.13 | 1.56 | 6.25 | 33 | 33 |
| Wine | > 50 | > 50 | > 50 | > 50 | > 50 | > 50 | > 50 | > 50 |
| Bile | > 50 | > 50 | > 50 | > 50 | > 50 | > 50 | > 50 | > 50 |
MICs were tested in both Mueller–Hinton Broth (MHB) or synthetic wound fluid (SWF). MICs are presented as modal values of 3 different batches (ESO 19–21; with 3 replicate MIC tests per batch) and are the percentage of treatment present at MIC (v/v).
Table 2.
Minimum inhibitory concentration (MIC) of ESO or preparations omitting a single ingredient.
| Treatment |
S. aureus Newman |
S. aureus USA300 |
A. baumannii clinical isolate |
P. aeruginosa PA14 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| MHB | SWF | MHB | SWF | MHB | SWF | MHB | SWF | ||
| ESO | < 0.78 | 12.5 | 3.13 | 12.5 | 1.56 | 6.25 | 25 | 25 | |
| Omitting | Onion | < 0.78 | 12.5 | 3.13 | 12.5 | 3.13 | 6.25 | 25 | 25 |
| Garlic | 50 | 50 | 50 | 50 | 50 | > 50 | > 50 | > 50 | |
| Wine | < 0.78 | 6.25 | 3.13 | 12.5 | 1.56 | 6.25 | 25 | 50 | |
| Bile | < 0.78 | 6.25 | 3.13 | 12.5 | 1.56 | 6.25 | 25 | 50 | |
MICs were tested in both Mueller–Hinton Broth (MHB) and synthetic wound fluid (SWF). MICs are presented as modal values of 3 different batches (ESO 22–24; with 3 replicate MIC tests per batch) and are the percentage of treatment present at MIC (v/v).
All four ingredients are necessary for activity against mature S. aureus biofilms in synthetic wounds
Consistent with previous results from our group, no single ingredient alone had any bactericidal activity against mature S. aureus Newman biofilms in the synthetic wound model, and removing any single ingredient resulted in a reduction in the anti-biofilm activity (Fig. 5). Surprisingly, removal of wine caused a decrease in activity on par with that seen with the removal of garlic, despite wine possessing very limited antimicrobial activity on its own in either biofilm or planktonic assays (Table 1). For comparison with planktonic MIC data, the final concentration of Bald’s eyesalve in synthetic wound biofilm killing assays (Figs. 1, 2, 3, 4, 5, 6), is 33% vol/vol. Therefore, in more in vivo-like biofilm conditions, combining all the ingredients is necessary for full activity against S. aureus Newman.
Figure 5.
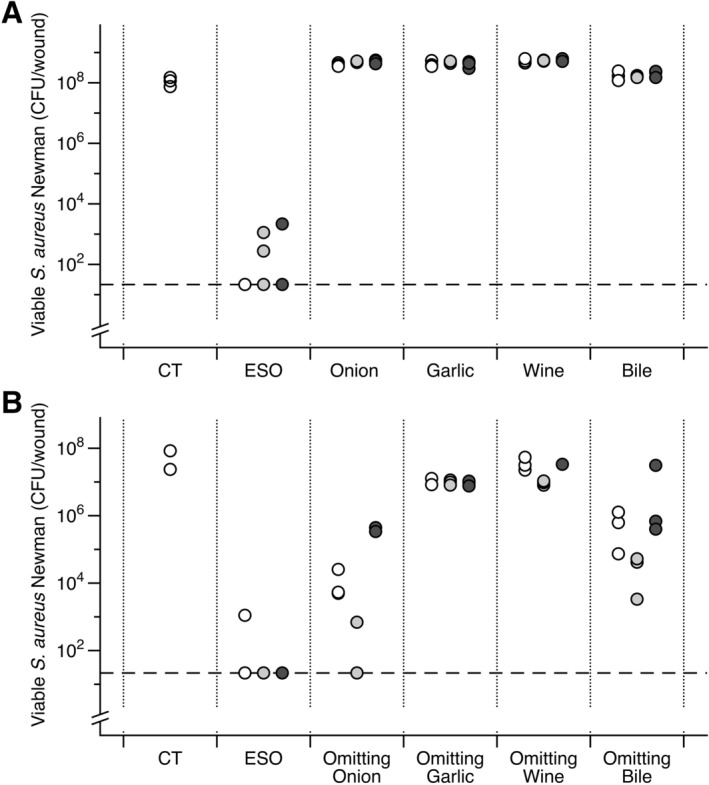
Contribution of ingredients to Bald’s eyesalve (ESO) anti-biofilm activity. Mature biofilms of S. aureus Newman, grown in a soft tissue wound model, were treated with water (CT) or treatment for 24 h, before recovering bacteria for CFU counts. Dashed lines represent the detection limit by plating. (A) ESO and individual ingredients, with the concentrations of each ingredient in the whole remedy and individual preparation being equal. Three batches of preparations are shown, ESO 19 (white), 20 (grey) and 21 (black); (n = 3 replica biofilms per treatment). Data was square-root transformed to meet assumptions of linear modelling. ANOVA showed significant differences between treatments (F4,30 = 389.215, p < 0.001), but not between batches (F2,30 = 1.24, p = 0.305) and the effect of treatment did not depend on the batch (interaction F8,30 = 0.930, p = 0.507). To compare each treatment to the untreated controls, we used a new ANOVA to fit least-squares means and variances for CFU recovered for each treatment (excluding controls), averaged over the three batches. Fitted means were compared with untreated controls using unpaired Welch’s t-tests. ESO treatment caused a significant decrease in CFU (t2.93 = 9.18, p = 0.003). Garlic, onion and wine caused small but significant increases (t2.93 = 9.05, p = 0.003, t2.93 = 9.61, p = 0.003, t2.93 = 11.1, p = 0.002), bile had no significant effect (t2.93 = 2.44, p = 0.094). (B) ESO and batches with one ingredient omitted and replaced with water. Three batches of preparations are shown, ESO 22 (white), 23 (grey) and 24 (black) (n = 3 replica biofilms per treatment). Data was log-transformed to meet the assumptions of linear modelling. ANOVA showed an effect of treatment (F4,26 = 162, p < 0.001) but not batch (F2,26 = 18.7, p < 0.001). The effect of treatment depended on the batch (interaction F8,26 = 5.85, p < 0.001), however, the rank order of treatments from least to most active were the same for each batch. As only two control data points were obtained, one-sample t-tests were performed to compare least-squares mean CFU in the treated wounds to the mean CFU in control wounds. A significant decrease in CFU was found for ESO and all dropouts (p ≤ 0.002) except the wine dropout (p = 0.202). Dunnett’s test revealed all dropouts were less active than the full ESO (all p < 0.001). Raw data and full statistical results are supplied in the Data Supplement.
Figure 6.
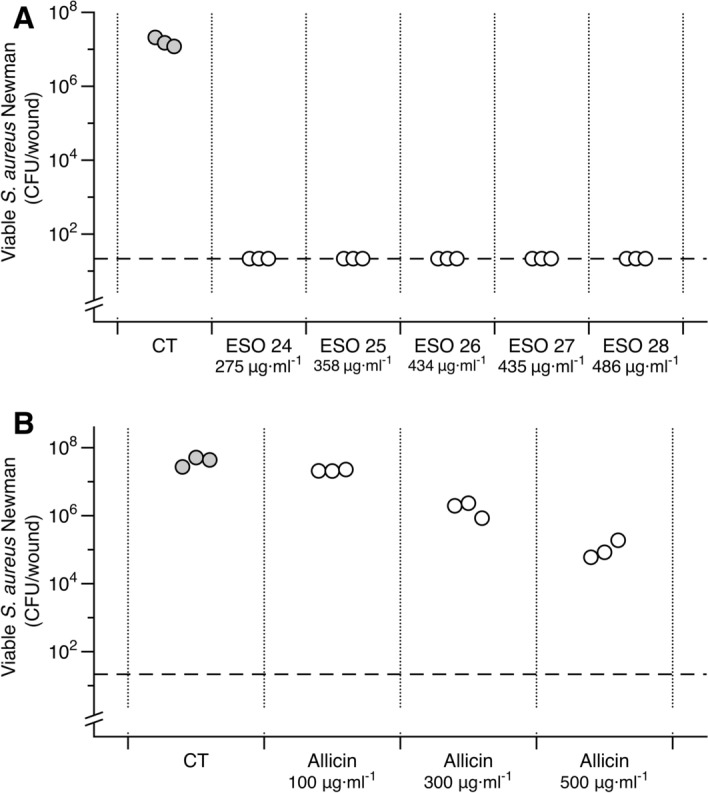
Antimicrobial activity of Bald’s eyesalve (ESO) and allicin standards. Mature S. aureus Newman biofilms were grown in a model of a soft tissue wound, then treated with either water (CT) or treatment for 24 h, before recovering bacteria for CFU counts. (A) Biofilms treated with 5 ESO batches, with their respective allicin concentrations indicated. (B) External allicin standards diluted in water. The dashed line represents the limit of detection by plating. To statistically analyse, data were log transformed to meet the assumptions of linear modelling. A one-way ANOVA was performed and found a significant difference between allicin treatments and control cells (F3,9 = 141, p < 0.001). A Dunnett’s test was conducted to compare the CFU of the three allicin concentrations with the CFU of the controls. Of the concentrations, 300 μg·ml−1 and 500 μg·ml−1 were significantly different (p < 0.001), 100 μg·ml−1 was not significantly different (p = 0.247). Raw data and full statistical results are supplied in the Data Supplement.
The antibacterial activity of ESO is not simply due to its allicin content
The study which attributed the planktonic activity of ESO to garlic further concluded that this was specifically due to the presence of allicin20, an organosulfur compound with well known, potent antimicrobial activity in vitro26–28. We used HPLC, calibrated against allicin standards of known concentrations, to determine the allicin concentration in 12 batches of fresh ESO. These batches were then assayed for MIC against S. aureus Newman in SWF. We were then able to calculate the concentration of allicin present in the MIC of each batch and compare this with the MIC of purified allicin.
As shown in Table 3, the allicin concentration in the 12 batches of ESO was found to be in the range of 391 ± 19 μg·ml−1. Therefore, the estimated concentration of allicin in their respective MICs would be in the range 11—39 μg·ml−1. This is much lower than the MIC obtained for purified allicin, which was 62.5 μg·ml−1. Due to experimental error, two MIC test (ESO batches 28, 29) were lost; we did not repeat this due to the time delay between the initial and repeated test potentially affecting concentrations of compounds present, and thus activity.
Table 3.
Minimum inhibitory concentrations (MIC) were calculated for fresh Bald’s eyesalve (n = 12; batches 19–30), garlic only preparations (n = 3) and external allicin standards (n = 3).
| Treatment | Allicin concentration range (mean ± s.e.) | Allicin concentration in MIC | Biofilm activity (log drop from control) | Average predicted allicin concentration in biofilm treatment |
|---|---|---|---|---|
| ESO | 275—486 (391 ± 19) μg·ml-1 | 11—39 μg·ml-1 | Eradicated biofilm (> 5) | 142 μg·ml-1 |
| Garlic only | 358—434 (389 ± 19) μg·ml-1 | 45—54 μg·ml-1 | No activity (0) | 160 μg·ml-1 |
| Allicin Standard | 62.5 μg·ml-1 | Partial activity (2.6) | 167 μg·ml-1 |
Allicin concentrations for each batch were quantified using HPLC and calculated using an allicin calibration curve. MICs were measured in synthetic wound fluid. The allicin concentration in the MIC of each batch, and the allicin concentration present in the biofilm treatment experiments, is calculated and the results of biofilm treatment experiments are provided for reference. Raw data is supplied in the Data Supplement.
All of these 12 batches eradicated mature S. aureus Newman biofilms in the synthetic wound assay (see Data Supplement). The results of biofilm killing assays for five representative batches are depicted in Fig. 6A, alongside a biofilm killing assay conducted for preparations of 100, 300 or 500 μg·ml−1 of purified allicin. Purified allicin at concentrations similar to those found in ESO, had much less anti-biofilm activity (Fig. 6B), and garlic preparations with the same allicin concentration as ESO had no activity (data supplied in the supplementary data file). Therefore, allicin alone does not explain either the activity of ESO against biofilm-grown bacteria or its activity against planktonic cultures.
Discussion
The activity of a range of natural products against microbes in simple in vitro assays (agar diffusion, broth microdilution or simple surface-attached biofilm assays) demonstrates their potential as a source of novel antibiotics29–31. However, the standard approach of purifying individual compounds from natural products rarely produces clinically useful products, and potent activity against planktonic bacteria in standard lab media rarely translates to in vivo efficacy. This is an especially pressing problem in the case of biofilm infections. Biofilms are much harder to treat due to reduced penetration of antibiotics through the extracellular matrix and the enhanced tolerance of biofilm-grown cells to many in-use antibiotics6,11. Biofilm infections of wounds (e.g. burns, diabetic foot ulcers), medical implants (e.g. artificial joints, catheters), the lungs (e.g. in cystic fibrosis) and other body sites impose a major health and economic burden32 and can be effectively untreatable. Non-healing, infected foot ulcers, which can be a complication of diabetes, provide an especially sobering example. Even if the infection is apparently successfully treated, there is a high chance of recurrence and an estimated 50% of those affected die within 5 years of ulcer development. Management of diabetic foot ulcers costs the UK’s NHS £650 M per year33.
Historical medical manuscripts often prescribe complex preparations of several ingredients to treat infections. Thus, when considering natural products as a potential source of anti-biofilm agents, we must consider the possibility that any efficacy they may possess could rely on creating a cocktail of different products. Understanding the relationship between combinations of natural products and antimicrobial activity may generate a novel way to create new antibiotics from botanicals. Here, we confirm Bald’s eyesalve as an example of an “ancientbiotic” that requires the combination of all ingredients for potent activity against a panel of clinically important bacterial strains.
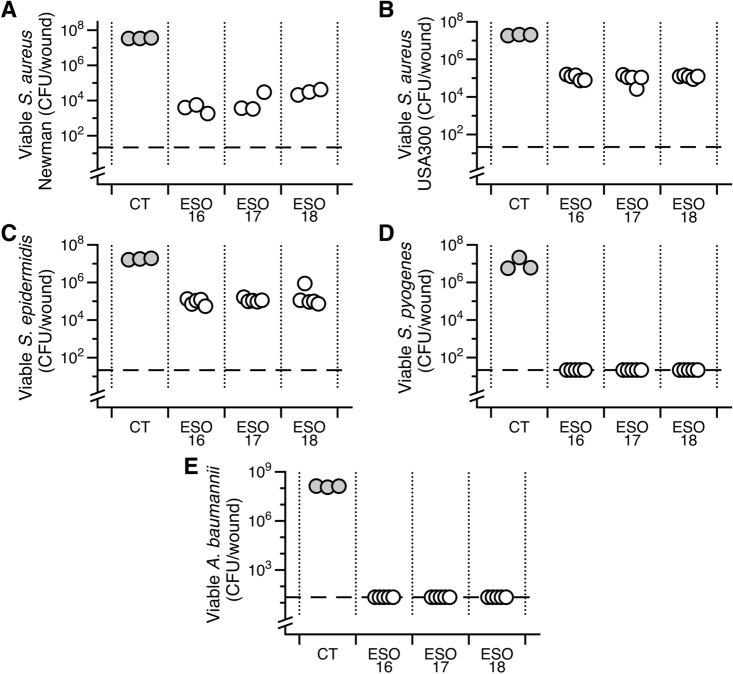
Our research builds on previous work19–21 to show that Bald's eyesalve can eradicate planktonic cultures of a range of problematic Gram-positive and Gram-negative bacteria including P. aeruginosa, A. baumannii, E. cloacae, S. maltophilia, S. aureus, S. epidermidis and S. pyogenes. It is also able to cause a 4-log reduction in viable cell counts in planktonic cultures of the especially problematic MRSA. Despite the widely understood problems of treating biofilms6,11, Bald’s eyesalve was also able to significantly reduce viable cell counts in biofilms of S. epidermidis and MRSA and was able to completely eradicate biofilms of S. aureus Newman, A. baumannii and S. pyogenes, in an established soft-tissue wound model24. However, although there was promising planktonic activity against P. aeruginosa, E. cloacae and S. maltophilia, variable or no activity was seen against biofilm cultures of these isolates in the wound model. This highlights the importance of investigating the anti-biofilm activity of candidate antibacterial agents, rather than extrapolating from the results of planktonic assays. Bald’s eyesalve shows great promise as an effective antimicrobial candidate, although further development and combination with biofilm-degrading adjuvants may be necessary to achieve activity against species such as P. aeruginosa. It would also be worthwhile to investigate potential anti-virulence effects of Bald’s eyesalve; given garlic’s ability to interfere with quorum sensing34,35 it is possible Bald’s eyesalve may usefully modulate bacterial behaviour when it does not kill36,37.
Each of Bald’s eyesalve ingredients has known antimicrobial properties or compounds (onion and garlic28,31,38, bile39–41, wine42–44). We explored the contribution of all four ingredients to both planktonic and biofilm activity of Bald’s eyesalve to build a picture of their relative contributions. Planktonic activity appeared almost entirely attributable to garlic. However, tests against S. aureus Newman biofilms, grown in a synthetic wound model, showed garlic exhibited no antibacterial activity in this more clinically-relevant setting. In fact, no preparation which omitted any one ingredient possessed full activity in the biofilm assay. This confirms our previously published finding that Bald’s eyesalve anti-biofilm activity is contingent on the presence of all four ingredients19.
Our results against planktonic cultures of S. aureus and P. aeruginosa align with a report by Fuchs et al. 2018, that garlic alone, and specifically allicin, accounts for the majority of the planktonic activity of Bald’s remedy. Allicin is a defensive compound that is converted from alliin by the enzyme alliinase, upon damage to the plant tissue. Allicin can kill a wide range of both Gram-negative and Gram-positive bacteria in vitro26 due to the thiosulfinate group (–S(O)–S–) reacting with many cellular proteins in the pathogen45. We therefore explored the role of allicin in Bald’s eyesalve in detail.
Crucially, we found that 12 batches of Bald’s eyesalve were able to elicit much greater anti-biofilm activity than purified allicin at comparable concentrations. Pure allicin at a concentration of 500 μg·ml−1 was able to reduce viable cell numbers by 2–3 logs, whereas Bald’s eyesalve batches containing 275–486 μg·ml−1 allicin were able to cause a 6–7 log drop and eradicate the biofilms. Even in planktonic tests, we found that the concentration of allicin in the MIC of these batches of Bald’s eyesalve was lower than the MIC of purified allicin. Together, these results clearly illustrate that in addition to allicin, other ingredients in Bald’s eyesalve contribute to its activity. This highlights the importance of the combination of ingredients.
The differences between our findings and those of Fuchs et al. are partly explained by the contrasts between bacterial tolerance to killing (i) in MHB versus synthetic wound fluid; and (ii) in planktonic culture versus established biofilms. This highlights the differences in antibiotic susceptibility often seen in host-mimicking media versus standard MHB17,18,46. Further, the allicin concentrations found in our batches of Bald’s eyesalve were lower than that found in Fuchs et al. The highest allicin concentration we measured was 486 μg·ml−1, compared with Fuchs et al.’s 836 μg·ml−1.
We think it unlikely that this is due to the differences in quantification methods used as both are appropriate. We used HPLC, a method that has been widely used previously27,47–50 and is accepted to produce consistent and accurate results. Previous papers using HPLC have detected allicin concentrations ranging from 1 μg·ml−1 to 2 mg·ml−1 27,49, a range that the results in this paper lie within. Fuchs et al. 2018 used quantitative NMR (qNMR), which too has advantages and is being increasingly used in quantification of concentrations51. It is more likely that our Bald’s eyesalve preparations really do have different allicin concentrations. Alliin is released from storage vesicles and converted to allicin upon damage to tissue: Fuchs et al., 2018 used a food processor, in comparison to our pestle and mortar, which may have resulted in greater damage to the tissue and therefore greater release of alliin and more allicin created. Further, different garlic varieties are thought to have different activities and compositions52–56 and our research labs are based in different continents, which presumably results in different garlic varieties and growth conditions. The higher allicin concentration may explain the slightly increased planktonic activity seen in Fuchs et al. This may also be due to their use of agitation in MIC assays, as agitation is known to lower the MIC57.
The step in the early Medieval remedy which specifies that the onion and garlic be ground together would have been conducted manually—most likely with a pestle and mortar. We have found that this process generates sub-bactericidal concentrations of allicin, which is complemented or synergised by the presence of other ingredients to form a biofilm-killing preparation. This demonstrates how contingent explorations of natural product antimicrobials are on material preparation and testing conditions. This result is also interesting because allicin can be toxic in high concentrations58. Reducing the amount of allicin present but maintaining full activity against bacterial biofilms may be key to producing a safe topical treatment. Further work is needed to elucidate the exact combination of natural products responsible for the anti-biofilm activity.
Contrary to many papers in the literature42,59,60, we found that wine possessed very limited planktonic antimicrobial activity, and could not kill S. aureus in biofilms. Despite this, its absence from the full remedy causes a large drop in activity against S. aureus biofilms. Taken together, these observations suggest that the role of wine in the full recipe may be more to do with its physical properties, perhaps its ethanol content and/or low pH. Ethanol is a well-known extraction solvent and may allow better extraction of compounds from the plant matter61, or allow better diffusion through biofilms, while the lower pH may activate pH-dependent compounds. The answer may lie in the combination of these properties, as the activity of wine reported in the literature cannot be attributed exclusively to one aspect of it: the physicochemical environment plays a large role in the activity of wine62. Although pH and ethanol concentrations corresponding to those found in various wines have minimal effects on pathogens, without them the compounds within the wine have reduced activity62.
Similarly, individual preparations of onion and bile, prepared as they would be for Bald’s eyesalve, possessed no planktonic antibacterial activity. This is contrary to the literature that has shown these ingredients to have activity against various strains39,63. The most likely cause for this disparity is the difference in the preparation or the dilution effect when combined with other ingredients. However, the removal of onion or bile significantly reduced the anti-biofilm activity against S. aureus, indicating the importance of their presence within the whole remedy. They may be providing additional compounds necessary for the full killing effect in biofilms or aiding penetration/activity of antibacterial compounds from other ingredients.
Here, we have shown for the first time that Bald’s eyesalve has anti-biofilm activity against several clinically relevant bacteria, and we confirm our earlier work which concluded that this activity is dependent on combining all the ingredients. Interestingly, statistical analysis of the surviving portion of Bald’s Leechbook (London, British Library, Royal MS 12 D XVII) showed that garlic is combined with a second Allium species significantly more often than would be expected given the frequency of use of either garlic or other Allium species across the book if ingredients were combined randomly64. Perhaps the patterns of ingredient combinations used by pre-modern physicians do, in at least some cases, reflect a requirement for combinatorial activity of several natural products to produce an efficacious antimicrobial preparation.
Research into natural products is often focussed on isolating single compounds, however, here we provide evidence that in doing so potent anti-biofilm mixtures can be overlooked. Viewing natural products in this way has the potential to open a vast new source of antimicrobials that can overcome the inherent difficulties of treating biofilm infections. In a companion manuscript21, we describe the low potential of Bald’s eyesalve for producing irritation or impeding wound healing. Future work will determine whether our results translate into a candidate natural product cocktail for incorporation into wound ointments or dressings. At present, we conclude by re-stating the exciting potential for pre-modern European medical texts to contain antibacterial preparations of clinical interest.
Materials and methods
Bacterial strains and culture conditions
Strains used are listed in Table 4. All cultures were grown aerobically at 37 °C and Miller’s LB agar (Melford) was used for plating.
Table 4.
Bacterial strains used in the study.
| Species | Strain | Notes |
|---|---|---|
| Gram-positive | ||
| Staphylococcus aureus | Newman | Standard laboratory strain, used in initial activity testing of all fresh eyesalve batches |
| Staphylococcus aureus | USA300 Los Angeles County clone | Well-studied example of community-acquired MRSA |
| Staphylococcus aureus | 6 human carriage isolates | 1 MRSA + 5 MSSA, gifted by Ruth Massey |
| Staphylococcus aureus | 6 clones isolated from a chronic post-surgical wound | MSSA, with thanks to Tim Sloan |
| Staphylococcus epidermidis | 1,457 | Standard laboratory strain, with thanks to Kim Hardie |
| Streptococcus pyogenes | Clinical isolate | Nottingham University Hospitals biobank, strain ref. 15W905066 |
| Gram-negative | ||
| Acinetobacter baumannii | Clinical isolate | Nottingham University Hospitals biobank, strain ref. 12W125768 |
| Enterobacter cloacae | Clinical isolate | Nottingham University Hospitals biobank, training isolate |
| Escherichia coli | K12 | Standard laboratory strain |
| Pseudomonas aeruginosa | PA14 | Standard laboratory strain |
| Stenotrophomonas maltophilia | Clinical isolate | Nottingham University Hospitals biobank, training isolate |
Media
Synthetic wound fluid (SWF) comprised equal volumes of foetal bovine serum (Gibco) and peptone water (Sigma-Aldrich)24. Mueller–Hinton Broth (MHB) was obtained from VWR. HyClone water (GE Healthcare) was used throughout.
Bald’s eyesalve reconstruction
Bald’s eyesalve was prepared as previously reported19. Garlic and onions were purchased from supermarkets or greengrocers. As lab work continued throughout all seasons of the year, and was conducted in two locations (Warwick and Nottingham), it is possible that different varieties of garlic and onion, or the same variety grown in different locations, were used in different batches of the eyesalve. The outer skin of the garlic and onion (sourced from local greengrocers) was removed. The garlic and onion were finely chopped, and equal volumes of garlic and onion were crushed together using a mortar and pestle for 2 min. Various sized batches of Bald’s eyesalve were used throughout this paper, ranging from final volumes of 30 ml–400 ml, the average weight used was 14.1 ± 1.5 g of onion and 15.0 ± 1.3 g of garlic per 100 ml of Bald’s eyesalve.
The crushed onion and garlic were then combined with equal volumes of wine (Pennard’s organic dry white, 11% ABV, sourced from Avalon Vineyard, Shepton Mallet) and bovine bile salts (Sigma Aldrich) made up to 89 mg·ml−1 in water and sterilised by exposing to UV radiation for 10 min (Carlton Germicidal Cabinet fitted with a 2537 Å, 8-W UV tube). The mixture was stored in sterilised glass bottles in the dark at 4 °C for 9 days, after which it was strained and centrifuged for 5 min at 1,811 g. The supernatant was then filtered using Whatman 1,001–110 Grade 1 Qualitative Filter Paper, Diameter: 11 cm, Pore Size: 11 μm. Filtered Bald’s eyesalve was stored in sterilised glass vials in the dark at 4 °C.
For ease of reading, batches are numbered as they appear within the paper. The key for their batch name is provided in the Data Supplement.
Preparation of individual ingredients and dropouts
Individual ingredients were prepared such that their concentrations were equal to concentrations present in the full remedy, a schematic is provided in Fig. S1. To achieve comparable concentrations, each ingredient was prepared as it would be for the full remedy (onion and garlic crushed separately from each other), with all other ingredients substituted with the same volume of water. A similar process was followed for “dropout” batches, where one ingredient at a time was systematically excluded from the remedy and replaced with an equal volume of water to maintain comparable concentrations to the original remedy.
Planktonic killing assay
Bacteria were cultured aerobically at 37 °C on LB agar for 18–24 h. Several colonies were then inoculated into 5 ml SWF and incubated for 6 h at 37 °C on an orbital shaker. Aliquots of each bacterial culture (100 μl) were added to wells of Corning Costar TC-Treated 96-Well Plate and 50 μL of the eyesalve batch to be tested was added to 5 wells per strain. 50 μL of water was used as a negative control. Plates were incubated in Tecan SPARK 10 M at 37 °C for 18 h with periodic orbital shaking (10 s at 20 min intervals). Serial dilutions were then performed and plated on LB agar plates.
Minimum inhibitory concentration assay by broth microdilution
The minimum inhibitory concentrations (MIC) of eyesalve, single ingredients, dropout batches and allicin were determined as described by Wiegand, Hilpert and Hancock (2008) in MHB and in SWF. Treatments, or water as a negative control, were serially diluted with media in Corning Costar TC-Treated 96-Well Plates, following the scheme in Table 5. Bacterial isolates were streaked onto LB agar to obtain single colonies. After 18–24 h incubation at 37 °C, three to five morphologically similar colonies were transferred to phosphate-buffered saline (PBS) and diluted to 0.5 McFarland standard (OD600 0.08–0.1). This suspension was diluted 1 in 100 in media, resulting in 5 × 105 CFU/ml. The bacterial suspension was then added to the treatment dilutions, resulting in another twofold dilution. The highest final concentration of eyesalve, single ingredient or dropout batch tested was 50%. Plates were incubated for 18 h at 37 °C. Results were visually checked for turbidity, and MIC values are the lowest concentration where growth was not visible.
Table 5.
Final volumes and calculated percentages of Bald’s eyesalve in wells of 96-well plates used for MIC.
| Well | Bald’s eyesalve (μl) | Media (μl) | Bacterial suspension (μl) | Final volume (μl) | % ESO in well |
|---|---|---|---|---|---|
| A | 50 | 0 | 50 | 100 | 50 |
| B | 33 | 17 | 50 | 100 | 33 |
| C | 25 | 25 | 50 | 100 | 25 |
| D | 12.5 | 37.5 | 50 | 100 | 12.5 |
| E | 6.25 | 43.75 | 50 | 100 | 6.25 |
| F | 3.13 | 46.87 | 50 | 100 | 3.13 |
| G | 1.56 | 48.44 | 50 | 100 | 1.56 |
| H | 0.78 | 49.22 | 50 | 100 | 0.78 |
Biofilm killing assay in synthetic wounds
Biofilms were created in a synthetic soft-tissue wound model as described by Werthén et al. (2010; 24) and as used in our previous work 19. Briefly, synthetic wounds were created on ice and comprised 2 mg·ml−1 collagen, 0.01% acetic acid, 60% [vol/vol] SWF, 10 mM sodium hydroxide. Synthetic wounds were incubated at 37 °C for 1 h to allow collagen to polymerise and placed under UV light for 10 min to ensure no contamination. Wounds were either 400 μl in 24-well culture plates or 200 μl in 48-well plates.
Bacteria were incubated aerobically in SWF on an orbital shaker for 6 h at 37 °C, after which cultures were diluted to OD600 of 0.1–0.2 with fresh SWF. Bacteria suspension was added to each synthetic wound (100 μl in 400 μl wounds or 50 μl in 200 μl wounds) and incubated at 37 °C for 24 h to allow biofilm formation.
Wounds containing mature biofilms were then exposed to Bald’s eyesalve or water (200 μl in 400 μl wounds or 100 μl in 200 μl wounds). Bacteria were recovered by treating with 600 μl (400 μl wounds) or 300 μl (200 μl wounds) of 0.5 mg·ml−1 collagenase type 1 (EMD Millipore Corp, USA) for 1 h at 37 °C to break down the matrix; the resulting liquid was serially diluted and plated on LB plates. Plates were incubated at 37 °C overnight, colonies were counted, and CFU/wound calculated.
High-performance liquid chromatography quantification of allicin
Allicin is an unstable and reactive compound under gas chromatography settings65, therefore, high-performance liquid chromatography (HPLC) was used to determine allicin concentrations65.
Various preparations of Bald’s eyesalve were analysed by reversed-phase HPLC on an Agilent 1,200 series system fitted with an Agilent ZORBAX Eclipse XDB-C18, 150 × 4.6 mm, 5 μm particle size column and diode array detection at 210 nm. A gradient of methanol (5–95%) in water was used at a flow rate of 1 ml·min−1 over 30 min. Injection volume was 10 μl, and the column temperature was 25 °C.
To identify the concentration of allicin in eyesalve preparations, a calibration curve was prepared by serially diluting an external allicin standard (Abcam, Cambridge) in water (twofold dilutions, from 750 μg·ml−1 to 0.5 μg·ml−1). External standards were run through HPLC as described above (n = 2) and allicin peak area, in the 210 nm reading, was plotted against concentration (μg·ml−1) (Fig. S2 and Data Supplement). Allicin external standards had a retention time of approximately 15 min (Fig. S2). This peak was confirmed in Bald’s eyesalve by comparing fresh eyesalve to the same batch spiked with additional allicin standard. The suspected peak increased as expected (Fig. S2).
Statistics
Data were analysed in R v3.5.1 (R Core Team, 2018) using the car66, lsmeans67, multcomp68 and FSA69 packages. Raw data and R code for all experiments are provided in the Data Supplement. Graphs were produced with DataGraph 4.5.1 (Visual Data Tools Inc. 2006–2020).
Supplementary information
Acknowledgements
We thank Mathew Diggle, Kim Hardie, Ruth Massey and Tim Sloan for bacterial strains; Meera Unnikrishnan for helpful comments on an early draft of the manuscript and the two anonymous reviewers for their helpful feedback on the manuscript; Callum Parsons, Colman Ó Cathail, Jason Millington and Thorulf Vargsen for pilot work; Callum Parsons, Jenny Littler, Shanjini Subhaskaran, Jason Millington, Thorulf Vargsen, Esther Sweeney and Colman O'Cathail for preparation of the eyesalve batches summarised in Fig. 1; and our colleagues who helped initiate work on this project – Steve Diggle, Aled Roberts, Kendra Rumbaugh and Rebecca Gabrilska. We also thank Cerith Harries, Caroline Stewart and the University of Warwick Media Preparation team for preparing the media used for this work. This work was supported by a Diabetes UK Project Grant to FH (ref. 17/0005690) and Jessica Furner-Pardoe is funded by the MRC Doctoral Training Partnership [grant number MR/N014294/1].
Author contributions
Conceived the study: C.L., F.H. Formulated hypotheses and designed experiments: J.F.P., B.O.A., R.C., J.M., C.A.O., D.A.B., C.C., F.H. Conducted experimental work: J.F.P., B.O.A., R.C., F.H. Analysed data: J.F.P., B.O.A., F.H. Drafted the manuscript: J.F.P., B.O.A. Contributed to manuscript preparation: R.C., J.M., C.A.O., C.L., D.A.B., C.C., F.H.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jessica Furner-Pardoe and Blessing O. Anonye.
Contributor Information
Jessica Furner-Pardoe, Email: j.furner-pardoe.1@warwick.ac.uk.
Freya Harrison, Email: f.harrison@warwick.ac.uk.
Supplementary information
is available for this paper at 10.1038/s41598-020-69273-8.
References
- 1.Teillant A, Gandra S, Barter D, Morgan DJ, Laxminarayan R. Potential burden of antibiotic resistance on surgery and cancer chemotherapy antibiotic prophylaxis in the USA: A literature review and modelling study. Lancet Infect. Dis. 2015;15:1429–1437. doi: 10.1016/S1473-3099(15)00270-4. [DOI] [PubMed] [Google Scholar]
- 2.O’Neill, J. Tackling drug-resistant infections globally : final report and recommendations the review on antimicrobial resistance. (2016).
- 3.Wright GD. Opportunities for natural products in 21st century antibiotic discovery. Nat. Prod. Rep. 2017;34:694–701. doi: 10.1039/c7np00019g. [DOI] [PubMed] [Google Scholar]
- 4.Guest JF, et al. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open. 2015;5:9283. doi: 10.1136/bmjopen-2015-009283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guest JF, et al. Health economic burden that different wound types impose on the UK’s National Health Service. Int. Wound J. 2017;14:322–330. doi: 10.1111/iwj.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Penesyan A, Gillings M, Paulsen IT. Antibiotic discovery: Combatting bacterial resistance in cells and in biofilm communities. Molecules. 2015;20:5286–5298. doi: 10.3390/molecules20045286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceri H, et al. The calgary biofilm device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999;37:1771–1776. doi: 10.1128/jcm.37.6.1771-1776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Høiby N, et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 2015;21:1–25. doi: 10.1016/j.cmi.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Tacconelli, E., Carrara, E., Savoldi, A., Kattula, D. & Burkert, F. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. (2019).
- 10.Pendleton JN, Gorman SP, Gilmore BF. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti. Infect. Ther. 2013;11:297–308. doi: 10.1586/eri.13.12. [DOI] [PubMed] [Google Scholar]
- 11.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;80(284):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 12.Benkeblia N. Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum) LWT Food Sci. Technol. 2004;37:263–268. [Google Scholar]
- 13.Chen C, et al. Broad-spectrum antimicrobial activity, chemical composition and mechanism of action of garlic (Allium sativum) extracts. Food Control. 2018;86:117–125. [Google Scholar]
- 14.Hooper SJ, Lewis MAO, Wilson MJ, Williams DW. Antimicrobial activity of CitroxTM bioflavonoid preparations against oral microorganisms. Br. Dent. J. 2011;210:1–5. doi: 10.1038/sj.bdj.2010.1224. [DOI] [PubMed] [Google Scholar]
- 15.Elfawal MA, et al. Dried whole plant artemisia annua as an antimalarial therapy. PLoS ONE. 2012;7:1. doi: 10.1371/journal.pone.0052746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elfawal MA, Towler MJ, Reich NG, Weathers PJ, Rich SM. Dried whole-plant artemisia annua slows evolution of malaria drug resistance and overcomes resistance to artemisinin. Proc. Natl. Acad. Sci. USA. 2015;112:821–826. doi: 10.1073/pnas.1413127112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubicek-Sutherland JZ, et al. Host-dependent induction of transient antibiotic resistance: A prelude to treatment failure. EBioMedicine. 2015;2:1169–1178. doi: 10.1016/j.ebiom.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ersoy SC, et al. Correcting a fundamental flaw in the paradigm for antimicrobial susceptibility testing. EBioMedicine. 2017;20:173–181. doi: 10.1016/j.ebiom.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison F, et al. A 1,000-year-old antimicrobial remedy with antistaphylococcal activity. MBio. 2015;6:1–7. doi: 10.1128/mBio.01129-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuchs AL, et al. Characterization of the antibacterial activity of Bald’s eyesalve against drug resistant Staphylococcus aureus and Pseudomonas aeruginosa. PLoS ONE. 2018;13:e0208108. doi: 10.1371/journal.pone.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anonye BO, et al. The safety profile of Bald’s eyesalve for the treatment of bacterial infections. BioRXiv. 2020 doi: 10.1101/2020.04.23.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bierbaumer, P., Sauer, H., Klug, H. W. & Krischke, U. Dictionary of Old English Plant Names. (2009). Available at: https://oldenglish-plantnames.org. (Accessed: 18th December 2019)
- 23.diPaolo Healey, A., Price Wilkin, J. & Xiang, X. Dictionary of Old English Web Corpus. Toronto: Dictionary of Old English Project 2009 (2009). Available at: https://tapor.library.utoronto.ca/doecorpus/. (Accessed: 18th December 2019)
- 24.Werthén M, et al. An in vitro model of bacterial infections in wounds and other soft tissues. Apmis. 2010;118:156–164. doi: 10.1111/j.1600-0463.2009.02580.x. [DOI] [PubMed] [Google Scholar]
- 25.Brenner VC, Sherris JC. Influence of different media and bloods on results of diffusion antibiotic susceptibility tests. Antimicrob. Agents Chemother. 1972;1:116–122. doi: 10.1128/aac.1.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999;1:125–129. doi: 10.1016/s1286-4579(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 27.Fujisawa H, et al. Antibacterial potential of garlic-derived allicin and its cancellation by sulfhydryl compounds. Biosci. Biotechnol. Biochem. 2009;73:1948–1955. doi: 10.1271/bbb.90096. [DOI] [PubMed] [Google Scholar]
- 28.Marchese A, et al. Antifungal and antibacterial activities of allicin: A review. Trends Food Sci. Technol. 2016;52:49–56. [Google Scholar]
- 29.Salam AM, Quave CL. Opportunities for plant natural products in infection control. Curr. Opin. Microbiol. 2018;45:189–194. doi: 10.1016/j.mib.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ta CAK, Arnason JT. Mini review of phytochemicals and plant taxa with activity as microbial biofilm and quorum sensing inhibitors. Molecules. 2016;21:2. doi: 10.3390/molecules21010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanzotti V, Bonanomi G, Scala F. What makes Allium species effective against pathogenic microbes? Phytochem. Rev. 2013;12:751–772. [Google Scholar]
- 32.Wolcott RD, et al. Chronic wounds and the medical biofilm paradigm. J. Wound Care. 2010;19:45–53. doi: 10.12968/jowc.2010.19.2.46966. [DOI] [PubMed] [Google Scholar]
- 33.National Institute for Health and Care Excellence. NICE guideline NG19: Diabetic foot problems: prevention and management. (2015). Available at: https://www.nice.org.uk/guidance/ng19. (Accessed: 26th February 2020) [PubMed]
- 34.Bodini SF, Manfredini S, Epp M, Valentini S, Santori F. Quorum sensing inhibition activity of garlic extract and p-coumaric acid. Lett. Appl. Microbiol. 2009;49:551–555. doi: 10.1111/j.1472-765X.2009.02704.x. [DOI] [PubMed] [Google Scholar]
- 35.Bjarnsholt T, et al. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology. 2005;151:3873–3880. doi: 10.1099/mic.0.27955-0. [DOI] [PubMed] [Google Scholar]
- 36.Cech NB, Junio HA, Ackermann LW, Kavanaugh JS, Horswill AR. Quorum quenching and antimicrobial activity of goldenseal (Hydrastis canadensis) against methicillin-resistant staphylococcus aureus (MRSA) Planta Med. 2012;78:1556–1561. doi: 10.1055/s-0032-1315042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ettefagh KA, Burns JT, Junio HA, Kaatz GW, Cech NB. Goldenseal (Hydrastis canadensis L.) extracts synergistically enhance the antibacterial activity of berberine via efflux pump. Planta Med. 2011;77:835–840. doi: 10.1055/s-0030-1250606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azu N, Onyeagba R. Antimicrobial properties of extracts of Allium cepa (Onions) and Zingiber officinale (Ginger) on Escherichia coli, Salmonella typhi and Bacillus subtilis. Internet J. Trop. Med. 2007;3:1–10. [Google Scholar]
- 39.Begley M, Gahan CGM, Hill C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005;29:625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Sung JY, Costerton JW, Shaffer EA. Defense system in the biliary tract against bacterial infection. Dig. Dis. Sci. 1992;37:689–696. doi: 10.1007/BF01296423. [DOI] [PubMed] [Google Scholar]
- 41.Sannasiddappa TH, Lund PA, Clarke SR. In vitro antibacterial activity of unconjugated and conjugated bile salts on Staphylococcus aureus. Front. Microbiol. 2017;8:1–11. doi: 10.3389/fmicb.2017.01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodríguez Vaquero MJ, Alberto MR, Manca Nadra MC. Influence of phenolic compounds from wines on the growth of Listeria monocytogenes. Food Control. 2007;18:587–593. [Google Scholar]
- 43.Rodríguez Vaquero MJ, Alberto MR, de Nadra MCM. Antibacterial effect of phenolic compounds from different wines. Food Control. 2007;18:93–101. [Google Scholar]
- 44.Carneiro A, Couto JA, Mena C, Queiroz J, Hogg T. Activity of wine against Campylobacter jejuni. Food Control. 2008;19:800–805. [Google Scholar]
- 45.Müller A, et al. Allicin induces thiol stress in bacteria through S-allylmercapto modification of protein cysteines. J. Biol. Chem. 2016;291:11477–11490. doi: 10.1074/jbc.M115.702308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imani Rad H, Arzanlou M, Ranjbar Omid M, Ravaji S, Peeri DH. Effect of culture media on chemical stability and antibacterial activity of allicin. J. Funct. Foods. 2017;28:321–325. [Google Scholar]
- 47.Bose S, Laha B, Banerjee S. Quantification of allicin by high performance liquid chromatography-ultraviolet analysis with effect of post-ultrasonic sound and microwave radiation on fresh garlic cloves. Pharmacogn. Mag. 2014;10:S288–S293. doi: 10.4103/0973-1296.133279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujisawa H, et al. Biological and chemical stability of garlic-derived allicin. J. Agric. Food Chem. 2008;56:4229–4235. doi: 10.1021/jf8000907. [DOI] [PubMed] [Google Scholar]
- 49.Fujisawa H, Suma K, Origuchi K, Seki T, Ariga T. Thermostability of allicin determined by chemical and biological assays. Biosci. Biotechnol. Biosci. 2008;72:2877–2883. doi: 10.1271/bbb.80381. [DOI] [PubMed] [Google Scholar]
- 50.Bocchini P, Andalò C, Pozzi R, Galletti GC, Antonelli A. Determination of diallyl thiosulfinate (allicin) in garlic (Allium sativum L.) by high-performance liquid chromatography with a post-column photochemical reactor. Anal. Chim. Acta. 2001;441:37–43. [Google Scholar]
- 51.Li X, Hu K. Quantitative NMR studies of multiple compound mixtures. Annu. Rep. NMR Spectrosc. 2017;90:85–143. [Google Scholar]
- 52.Shobana S, Vidhya VG, Ramya M. Antibacterial activity of garlic varieties (Ophioscordon and Sativum) on enteric pathogens. Curr. Res. J. Biol. Sci. 2009;1:123–126. [Google Scholar]
- 53.Eagling, D. & Sterling, S. A cholesterol-lowering extract from GARLIC. (© 2000 Rural Industries Research and Development Corporation, 2000).
- 54.Bhandari SR, Yoon MK, Kwak JH. Contents of phytochemical constituents and antioxidant activity of 19 garlic (Allium sativum L.) parental lines and cultivars. Hortic. Environ. Biotechnol. 2014;55:138–147. [Google Scholar]
- 55.Beato VM, Orgaz F, Mansilla F, Montaño A. Changes in phenolic compounds in garlic (Allium sativum L.) owing to the cultivar and location of growth. Plant Foods Hum. Nutr. 2011;66:218–223. doi: 10.1007/s11130-011-0236-2. [DOI] [PubMed] [Google Scholar]
- 56.Fotopoulos, V. et al. Garlic, from Remedy to Stimulant: Evaluation of Antifungal Potential Reveals Diversity in Phytoalexin Allicin Content among Garlic Cultivars; Allicin Containing Aqueous Garlic Extracts Trigger Antioxidants in Cucumber. (2016). doi:10.3389/fpls.2016.01235 [DOI] [PMC free article] [PubMed]
- 57.Sawer IK, Berry MI, Ford JL. Effect of medium composition, agitation and the presence of EDTA on the antimicrobial activity of cryptolepine. Lett. Appl. Microbiol. 1997;25:207–211. doi: 10.1046/j.1472-765x.1997.00206.x. [DOI] [PubMed] [Google Scholar]
- 58.Gruhlke MCH, Nicco C, Batteux F, Slusarenko AJ. The effects of allicin, a reactive sulfur species from garlic, on a selection of mammalian cell lines. Antioxidants. 2017;6:1. doi: 10.3390/antiox6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friedman M. Antibacterial, antiviral, and antifungal properties of wines and winery byproducts in relation to their flavonoid content. J. Agric. Food Chem. 2014;62:6025–6042. doi: 10.1021/jf501266s. [DOI] [PubMed] [Google Scholar]
- 60.Møretrø T, Daeschel MA. Wine is bactericidal to foodborne pathogens. J. Food Sci. 2006;69:M251–M257. [Google Scholar]
- 61.Zhang QW, Lin LG, Ye WC. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018;13:1. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boban N, et al. Antimicrobial effects of wine: Separating the role of polyphenols, pH, ethanol, and other wine components. J. Food Sci. 2010;75:322–326. doi: 10.1111/j.1750-3841.2010.01622.x. [DOI] [PubMed] [Google Scholar]
- 63.Ahiabor C, Gordon A, Ayittey K, Agyare R. In vitro assessment of antibacterial activity of crude extracts of onion (Allium cepa L.) and shallot (Allium aescalonicum L.) on isolates of Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 25923), and Salmonella typhi (ATCC 19430) Int. J. Appl. Res. 2016;2:1029–1032. [Google Scholar]
- 64.Harrison, F. & Connelly, E. Could Medieval Medicine Help the Fight Against Antimicrobial Resistance? in Making the Medieval Relevant (eds. Jones, C., Kostick, C. & Oschema, K.) 113–134 (De Gruyter, 2019). doi:10.1515/9783110546316–005
- 65.Mondy N, Naudin A, Christides JP, Mandon N, Auger J. Comparison of GC-MS and HPLC for the analysis of Allium volatiles. Chromatographia. 2001;53:356–360. [Google Scholar]
- 66.Fox, J. & Weisberg, S. An R Companion to Applied Regression. (2011).
- 67.Lenth, R. V. Lsmeans: R Package. (2017).
- 68.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biometric. J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 69.Dinno, D. H. O. and P. W. and A. FSA: Fisheries Stock Analysis. (2020)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.