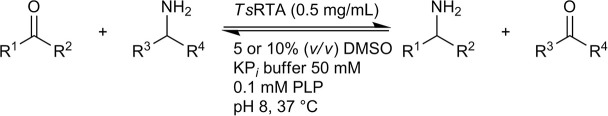
Table 2.
Carbonyl substrate scope (10 mM scale) of wild-type TsRTA, unless stated otherwisea.
 | ||||
|---|---|---|---|---|
| Substrate | Conversion (%) | Product ee (%) | ||
| After 30 min | After 24 h | |||
| Benzaldehyde |  |
79 ± 1 | 89 ± 2 | |
| Phenylacetaldehyde |  |
1.5 ± 0.8 | 2.5 ± 0.8 | |
| Cinnamaldehyde |  |
38 ± 1 | 60 ± 1 | |
| Vanillin |  |
16 ± 1 12 ± 1d |
72 ± 3 93 ± 3d |
|
| Butanone |  |
<0.5 | 12 ± 1 | >99.5 (R) |
| Hexan-2-oneb |  |
30 ± 1 30 ± 1d |
57 ± 1 70 ± 2d |
>99.5 (R) |
| Pinacolone |  |
<0.5 | 1.8 ± 0.8 | n.d. |
| 2,2-Dimethylhexan-3-one |  |
<0.5 | <0.5 | n.d. |
| Cyclohexanone |  |
<0.5 | 7.7 ± 0.7 | |
| Tetrahydrothiophene-3-oneb |  |
5.5 ± 0.7 | 32 ± 1 | 21 ± 1 (R) |
| Tetrahydrofuran-3-oneb | 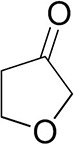 |
1.0 ± 0.8 | 25 ± 1 | 22 ± 1 (S) |
| Pyruvate |  |
75 ± 1 | 95 ± 1 | n.d. |
| β-Hydroxy-pyruvate |  |
52 ± 1 | 46 ± 1 | >99.5 (R) |
| α-Ketoglutarate |  |
<0.5 | <0.5 | n.d. |
| Acetophenoneb,c |  |
<0.5 | 4 ± 3 | traces (R) |
| o-Fluoroacetophenoneb |  |
34 ± 1 35 ± 2d |
70 ± 1 80 ± 3d |
>99.5 (R) |
| 1-Indanoneb |  |
1.8 ± 0.8 | 3.6 ± 0.8 | traces (R) |
| Propiophenoneb |  |
<0.5 | 1.2 ± 0.8 | traces (R) |
| Phenoxyacetoneb |  |
65 ± 1 50 ± 1d,e |
81 ± 4 95 ± 1d,e |
>99.5 (R) |
| 4-Phenylbutanoneb |  |
17 ± 1 13 ± 1d |
51 ± 3 75 ± 1d |
>99.5 (R) |
Unless otherwise stated, for the carbonyl acceptor scope, 10 mM RMBA was used as the amine donor. Conversions are based on the formation of acetophenone as determined by HPLC and calculated from a calibration curve. All reactions were carried out in triplicate. Standard errors include the SE of the calibration curve (Ellison and Williams, 2012; Theodorou et al., 2012). Any conversion <1% may be due to the exchange of PMP for PLP following the first half reaction with RMBA. Enantiomeric excesses were determined after 24 h by chiral GC-FID or chiral RP-HPLC (butanone, tetrahydrofuran-3-one, β-hydroxy-pyruvate).
10% (v/v) DMSO.
50 mM isopropylamine.
TsRTA_G205C.
0.1 mg/mL enzyme.
“ < 0.5” = calculated conversion smaller than uncertainty from calibration curve. “n.d.” = no product detected.
