Abstract
Background
Data on the occurrence of acute kidney injury (AKI) in patients undergoing cardiac resynchronization therapy (CRT) implantation is limited and no previous studies investigated its impact in an elderly population. CRT implantation requires a relatively low quantity of contrast medium. Previous studies, however, focused primarily on contrast medium as etiological factor for AKI, reporting a high incidence (8–14%). The high incidence of AKI in absence of use of substantial amounts of contrast volume, suggests the existence of other factors that contribute to AKI.
Objectives
To determine the predictive value of patient and procedure-related risk factors for the occurrence of AKI post CRT, as well as the AKIs impact on length of in-hospital stay (LOS) and 1-year mortality.
Methods
Retrospective observational study, including consecutive patients that underwent CRT implantation in a single center.
Results
60 patients with a mean age of 77 ± 8.4 years were included in the study and Twelve (20%) developed AKI. Prior renal insufficiency (p = 0.03; OR = 15.4), larger procedure time (p = 0.02; OR = 1.03), intra-operative hypotension (p < 0.01; OR = 1.72) and bleeding (p = 0.01 (OR = 7.86), showed to predict AKI significantly. AKI associated a significantly longer LOS (12 vs 3 days, p < 0.01). No significant differences regarding 1-year mortality were observed (p = 0.19; HR = 2.7 for patients with AKI).
Conclusions
AKI is a frequent complication of CRT implantation with an important impact on in-hospital stay, especially in the elderly. In addition to contrast administration, clinical factors could play a significant role in the occurrence of AKI.
Keywords: Cardiac resynchronization therapy, Acute kidney injury, Incidence, Predictors
1. Introduction
Cardiac resynchronization therapy (CRT) has shown to reduce hospitalization and mortality and to improve cardiac function through biventricular pacing, in patients with symptomatic chronic heart failure (HF) with optimal medical treatment, severely depressed left ventricular ejection fraction (LVEF) and complete left bundle branch block. Although only a small proportion of HF patients is eligible (an estimated 5–10%) for this treatment, this represents still a large absolute number of patients [1].
In order to achieve optimal placement of the left ventricular lead, a detailed assessment of coronary sinus anatomy is required, being coronary venous angiogram considered the gold standard [2]. Injections of intravascular iodinated contrast medium associate the risk of contrast-induced acute kidney injury (CI-AKI), a syndrome defined as an increase of serum creatinine of ≥25% or ≥0.5 mg/dl within 48 h after contrast administration. Furthermore, advanced congestive heart failure is one of the most important risk factors of CI-AKI [3].
Thus, the detailed evaluation of acute kidney injury (AKI) is crucial in patients undergoing CRT implantation. However, only a few studies have investigated the occurrence of AKI after CRT, concluding that this complication is possibly an under-recognized entity, with an incidence of up to 14%, which may have a negative impact on morbidity and mortality [4], [5], [6]. All these studies focused on the administration of contrast medium as primary etiological risk factor for AKI. CRT implantation requires substantially less administration of contrast medium, compared with other invasive procedures, such as percutaneous coronary intervention (PCI) [6]. Therefore, the high incidence of AKI post CRT implantation, as described by the previously mentioned studies, suggests the existence of other patient or procedure-related factors that may contribute to AKI occurrence. Furthermore, none of the above mentioned studies investigated the impact of AKI post TRC in an elderly population.
1.1. Objectives
This study aims to assess the incidence of AKI post CRT implantation and its impact on length of in-hospital stay (LOS) and 1-year mortality. Furthermore, we investigated the predictive value of patient and procedure-related risk factors for the occurrence of AKI, beyond the administration of contrast medium.
2. Methods
2.1. Patients
Consecutive patients that underwent CRT implantation in our hospital between April 2014 and May 2019 were included for retrospective chart review. Inclusion and exclusion criteria are detailed in Supplementary Table A.1.
2.2. Procedure details
Indication for CRT implantation was made according to the current European guidelines on Cardiac pacing and Cardiac resynchronization therapy. [1] Implantation was performed according to our center’s standard operating procedure, in agreement with standard transvenous techniques [2], [7], [8]. The procedure was carried out by a multidisciplinary team, including experienced interventional cardiologists, heart surgeons and anesthesiologists. Intra-operative non-invasive blood pressure was measured every 15 min and documented in the anesthesia record for all cases. We used standard, commercially available resynchronization systems (Quadra Assura; Abbott/St. Jude Medical, USA) and leads (Optisense, Durata, Quartet; Abbott/St. Jude Medical, USA) for pacing and sensing of the atrium, right ventricle and left ventricle.
All patients were assessed for risk of CI-AKI according to the Mehran score and received the following preoperative prevention measures: 1) Pre- and postoperative intravenous hydration with isotonic saline (0.5 ml/kg/h 12 h before and 24 h after the procedure). 2) Acetylcysteine, 600 mg twice daily (pre- and postoperative for a total of 4 doses), was used in patients with a high risk of CI-AKI according to the Mehran score. 3) Metformin, as well as potentially nephrotoxic drugs, such as NSAIDs and diuretics, were discontinued according to our standard pre-operative protocol [3], [9]. AKI was defined as an increase of serum creatinine of ≥25% or ≥0.5 mg/dl within 48 h after CRT implantation, in agreement with previous studies on AKI post CRT [3]. The estimated glomerular filtration rate (eGFR) was calculated using the 4-variable Modification of Diet in Renal Disease (MDRD) equation [10].
2.3. Contrast medium
Iomeprol 400, (Iomeron®, TiVo Corporations, Santa Clara, CA, USA, 50 ml per bottle), a low-osmolar, non-ionic contrast medium, was used in all procedures. Since increase of contrast induced acute kidney injury in patients undergoing CRT implantation is correlated with the use of >100 ml of contrast medium, cases in which >2 bottles of Iomeprol (50 ml, each) were registered in the surgery material chart, were excluded from the study [6].
2.4. Statistical analysis
The distribution of data was tested with the Kolmogorov-Smirnov test. Descriptive statistics were used to summarize data, presenting medians and ranges for continuous non-parametric data, whereas mean and standard deviation are depicted for parametric data. Proportions were analyzed by generating cross-tables, using chi-square test to determine significant differences. Independent groups of non-parametric data were compared using the Mann-Whitney U test.
Univariate and multivariate binary inclusive logistic regression models were generated in order to identify independent predictors of AKI. We tested the following independent variables to determine their predictive value for the occurrence of AKI: 1) Patient-related risk factors: a) pre-operative eGFR < 60 ml/min/1.73 m2, b) Creatinine on admission, c) Hemoglobin on admission, d) NT-proBNP on admission. 2) Procedure-related risk factors: a) Procedure time, b) Intraoperative hypotension (defined as drop of systolic blood pressure (SBP) of ≥20% with regard to SBP at start of procedure for at least 15 min), c) Intraoperative bleeding (defined as ≥2 g/dl decrease in hemoglobin concentration or necessity of red blood cell transfusion). Each multivariate regression model included the variables age, gender, NYHA functional class and LVEF on admission, as co-variates.
We used Log-rank tests to compare Kaplan-Meier curves plotted for cumulative survival to evaluate 12-month mortality (each for all causes and HF). Multivariate Cox regression models were generated in order to determine the predictive value of the independent variable AKI on mortality.
All statistical analyses were performed using IBM SPSS (v21.0).
2.5. Ethics
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a priori approval by the institution's human research committee.
3. Results
3.1. Patients’ characteristics
A total of 65 consecutive patients that underwent CRT implantation in the period between April 2014 and May 2019 were included in the study. Three patients were excluded due to epicardial approach and two patients were excluded because of exceptional high (>100 ml) use of contrast medium. The remaining 60 patients were included in further analysis. The mean age was 77 ± 8.4 years. Details concerning demographic data and clinical characteristics are shown in Table 1. No significant differences were found between patients that developed AKI and those who did not develop AKI, regarding demographic and clinical data. The median baseline eGFR was 57 ml/min/1.73 m2, and median baseline Creatinine was 1.15 mg/dl, with no significant differences between patients that developed AKI and those who did not develop AKI (p = 0.18 and 0.83, respectively).
Table 1.
Demographic data, clinical characteristics of overall population and of subgroups according to the occurrence of AKI.
| A: Overall Population | B: AKI,n = 12 | C: No AKI, n = 48 | P value (B vs C) | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age - years | 77.0 (SD 8.4) | 78.0 (SD 5.2) | 76.6 (SD 9.0) | 0.69 |
| Male gender – no (%) | 50 (83.3) | 9 (75) | 41 (85.4) | 0.38 |
| History of atrial fibrillation – no (%) | 36 (60) | 9 (75) | 27 (56.3) | 0.32 |
| Arterial hypertension – no (%) | 54 (90) | 11 (91.7) | 43 (89.6) | 0.83 |
| Diabetes mellitus – no (%) | 25 (41.7) | 5 (41.7) | 20 (41.7) | 1.00 |
| Prior CABG – no (%) | 5 (8.3) | 3 (25) | 2 (4.2) | 0.05 |
| Ischemic cardiomyopathy – no (%) | 39 (65) | 9 (75) | 30 (62.5) | 0.41 |
| NYHA class on admission | 3 (2–4) | 3 (2–4) | 3 (2–3) | 0.91 |
| Left ventricle ejection fraction - % | 31 (21–45 | 33 (21–40) | 30 (18–45) | 0.86 |
| NT-proBNP on admission - pg/mL | 6893 (800–20452) | 6991 (1727–19575) | 5325 (800–20452) | 0.37 |
| Medication | ||||
| Beta-adrenergic blocker – no (%) | 56 (93.3) | 12 (1 0 0) | 44 (91.7) | 0.57 |
| ACE-Inhibitor / ARB – no (%) | 53 (88.3) | 10 (83.3) | 43 (89.6) | 0.62 |
| Spironolactone/Epleronone – no (%) | 42 (70) | 9 (75) | 33 (68.8) | 0.67 |
| Loop diuretics – no (%) | 48 (80) | 10 (83.3) | 38 (79.2) | 0.74 |
| Digoxin – no (%) | 7 (11.7) | 1 (8.3) | 6 (12.5) | 0.68 |
| Statin – no (%) | 54 (90) | 11 (91.7) | 43 (89.6) | 0.83 |
| Neprilisin inhibitor – no (%) | 9 (15) | 2 (16.7) | 7 (14.6) | 0.85 |
| Biochemical analysis | ||||
| Creatinine on admission – umol/L | 1.15 (0.52–4.19) | 1.20 (0.52–1.98) | 1.12 (0.64–4.19) | 0.83 |
| GFR on admission – ml/min/1.73 m2 | 57 (14–102) | 51 (29–94) | 58 (14–102) | 0.18 |
| Hemoglobin on admission – g/dl | 12.8 (8–17.5) | 12.4 (9.3–15.6) | 12.9 (8–17.5) | 0.78 |
ACE Angiotensine converting enzyme, ARB angiotensin receptor blocker, CABG coronary artery by-pass grafting, AKI acute kidney injury, GFR glomerular filtration rate, NT-proBNP N-terminal prohormone of brain natriuretic peptide, NYHA New York Heart Association.
3.2. Procedure details
Mean procedure time was 114.5 min, being significantly higher in patients that developed AKI than in patients that did not develop AKI (135.9 vs 109.5 min respectively, p = 0.03). Mean SBP at the beginning of the intervention was 136.3 mmHg, with no significant differences between the two subgroups. Drop in SBP of ≥20% with regard to SBP at the beginning of the procedure occurred in 8 (16.3%) patients. Patients that presented intra-operative drop of SBP were significantly more likely to develop AKI than patients without SBP drop (62.5 vs 7.3%, respectively, p < 0.01). Intra-operative bleeding occurred in 9 (15%) patients, those being significantly more likely to develop AKI than patients that did not present this complication (55.6 vs 13.7%, respectively, p = 0.012). See Table 2 for details.
Table 2.
Procedure details and Outcomes of overall population and of subgroups according to the occurrence of AKI.
| A: Overall Population | B: AKI,n = 12 | C: No AKI, n = 48 | P value (B vs C) | |
|---|---|---|---|---|
| Procedure details | ||||
| Procedure time – min | 114.5 (SD 32.14) | 135.9 (SD 34.0) | 109.5 (SD 29.9) | 0.03 |
| SBP at the beginning of intervention – mmHg | 136.3 (SD 17.9) | 143.1 (SD 20.2) | 135.0 (SD 17.4) | 0.24 |
| Renal parameters | ||||
| Creatinine post OP - umol/L | 1.29 (0.66–3.85) | 1.76 (1.13–3.19) | 1.26 (0.66–3.85) | <0.01 |
| GFR post OP - ml/min/1.73 m2 | 40 (11–113) | 27 (14–113) | 41 (11–88) | <0.01 |
| Biochemical analysis | ||||
| Hemoglobin post OP – g/dl | 12.6 (7.3–13.9) | 11.6 (7.6–14.2) | 13.0 (7.3–13.9) | 0.05 |
| LOS and Mortality | ||||
| LOS – days | 3 (2–28) | 12 (4–28) | 3 (2–11) | < 0.001 |
| 12 month mortality (all cause) - % | 7 (11.7) | 3 (25) | 4 (8.3) | 0.10 |
| 12 month mortality (cardiac) - % | 3 (5) | 1 (8.3) | 2 (4.2) | 0.55 |
ACE Angiotensine converting enzyme, ARB angiotensin receptor blocker, CABG coronary artery by-pass grafting, AKI acute kidney injury, GFR glomerular filtration rate, LOS length of in-hospital stay, NT-proBNP N-terminal prohormone of brain natriuretic peptide, NYHA New York Heart Association, SBP systolic blood pressure.
3.3. Incidence of AKI and impact on LOS
Overall, 12 (20%) out of the 60 enrolled patients developed AKI; none of them required hemodialysis or hemofiltration. AKI was associated with a significantly longer LOS (median of 12 days in patients that developed AKI vs 3 days in patients without AKI, p < 0.01). Details are depicted in Table 2.
3.4. Predictors of AKI
3.4.1. Patient related risk factors
Occurrence of AKI was significantly higher in patients with eGFR < 60 ml/min/m2 at baseline than in patients without prior renal dysfunction (30.6% vs 4.2%, respectively, p = 0.01). See Table 1 for details. Furthermore, prior deterioration of the renal function showed to be a significant independent predictor for the occurrence of AKI, as determined by univariate and multivariate binary inclusive logistic regression models (p = 0.033 (OR 10.1), p = 0.026 (OR 15.4). The other patient-related independent variables (Creatinine, Hemoglobin and NT-proBNP on admission) did not predict AKI. Details are shown in Table 3.
Table 3.
Evaluation of independent predictors for the occurrence of AKI.
| Independent variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Prior deterioration of renal function* | 10.1 (1.3–15.8) | 0.033 | 15.4 (1.3–17.8) | 0.026 |
| Procedure time | 1.03 (1.05–3.5) | 0.011 | 1.03 (1.04–3.8) | 0.024 |
| Intra-operative drop in SBP of ≥20% | 1.77 (1.2–4.7) | 0.001 | 1.72 (1.1–5.1) | 0.003 |
| Intra-operative bleeding† | 7.86 (1.6–8.9) | 0.009 | 5.45 (0.95–6.6) | 0.081 |
CI Confidence interval, AKI acute kidney injury, GFR glomerular filtration rate, NT-proBNP N-terminal prohormone of brain natriuretic peptide, SBP systolic blood pressure.
Non-significant co-variates included in each regression model were: 1) Age on admission, 2) Gender, 3) LVEF on admission, 4) NYHA functional class on admission.
Defined as GFR < 60 ml/min/1.73 m2 on admission, †Defined as defined as ≥2 g/dl decrease in hemoglobin concentration or necessity of red blood cell transfusion.
3.4.2. Procedure related risk factors
A higher procedure time, resulted to be a significant predictor for AKI (p = 0.011 (OR: 1.03) and p = 0.024 (OR: 1.03), respectively). Univariate and multivariate regression models indicated that an intra-operative drop in SBP of ≥20% predicted occurrence of AKI (p = 0.001 (OR: 1.77) and p = 0.003 (OR: 1.72), respectively). Furthermore, intra-operative bleeding showed to be a significant predictor in univariate regression models, with a tendency towards statistical significance in multivariate regression models (p = 0.01 (OR: 7.86) and p = 0.08 (OR: 5.45), respectively). See Table 3 for details.
3.4.3. Interaction between co-variables
Generating a multivariate logistic regression model, including the variables that showed to be significant predictors of AKI, only prior deterioration of the renal function and intra-operative drop in SBP remained statistically significant (p = 0.048 and p = 0.012, respectively).
3.5. Impact on 1-year mortality
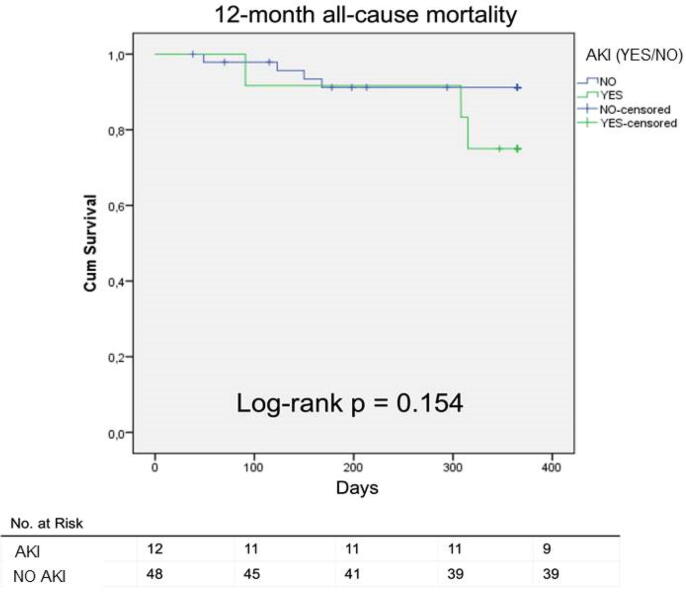
Cumulative surviving concerning 12-month all-cause mortality is presented in a Kaplan-Meier curve (Fig. 1). The differences in mortality (all-cause and cardiac) did not reach statistical significance, as determined by Log-rank test. Multivariate Cox-regression analysis that included demographic and clinical characteristics, such as age, gender, LVEF and NYHA functional class, evaluated the value of AKI for the prediction of mortality, presenting a hazard ratio of 2.7 for the outcome of 12-month all-cause mortality (p = 0.19) and 2.1 (p = 0.55) for cardiac mortality. See Table A.2 for details.
Fig. 1.
Kaplan-Meier curve presenting cumulative surviving concerning 12-month all-cause mortality.
4. Discussion
4.1. Study population and incidence of AKI
Our study demonstrates that acute kidney injury is a frequent complication of CRT implantation, which has a negative impact on LOS, especially in the elderly. CI-AKI has previously been described as a major complication with significant impact on long-term survival in patients undergoing coronary angiography and PCI [11], [12], [13]. Despite the abundance of studies, investigating the incidence of AKI in patients undergoing coronary angiography and PCI, there is only limited data available on the AKI occurrence after CRT implantation. Furthermore, no studies investigated the impact of AKI post TRC in an elderly population [4], [5], [6]. The patients’ mean age in our study was 77 ± 8.4 years, whereas Kowalczky et al. presented in their subanalysis of a randomized trial a significantly younger group of patients (61 (IQR: 39–82) years). This sub-analysis of the TRUST CRT trial revealed a 10.2% incidence (however, applying a different definition of AKI: rise in serum creatinine of at least 26.5 μmol/L (0.3 mg/dL) within 48 h after contrast exposure, or at least 50% increase from the baseline value during index hospital stay), whereas in a study by Cowburn et al. AKI occurred in 14% of patients that underwent CRT implantation [4], [5], [6]. In comparison to the previously mentioned studies, we found a higher incidence of AKI (20%). This may be explained by the significantly older and therefore more vulnerable study population, which is underlined by a worse baseline clinical status of our study population (high prevalence of patients with GFR < 60 ml/min/m2 on admission (60%) and higher NT-proBNP on admission).
4.2. Impact of AKI on LOS
Furthermore, we showed that LOS was significantly longer in patients that developed AKI than in patients that did not present this complication. This finding is in line with previous findings of Cowburn et al. and stands in contrast to the results of the TRUST CRT trial, where no significant difference could be found [4], [5]. Future prospective studies are needed to confirm the negative impact of AKI on the length of hospital stay.
4.3. Predictors of AKI
CRT implantation usually requires substantially less administration of contrast medium than PCI. Therefore, the high incidence of AKI post CRT implantation, as described by the previously mentioned studies, suggests the existence of other patient or procedure-related factors that may contribute to AKI occurrence. Nevertheless, all previous studies investigating AKI after CRT implantation focused primarily on the administration of contrast medium as the main etiological risk factor. Our study, on the other hand, investigated the predictive value of patient and procedure-related risk factors. We present an elderly study population, in which the prevalence and impact of these factors may be even more important than in a younger subgroup of patients.
4.3.1. Patient related risk factors
Pre-existing renal impairment, as defined as GFR < 60 ml/min/m2, showed to be a significant independent predictor for the occurrence of AKI in patients that underwent CRT-implantation. This finding is in line with previous trials on CI-AKI risk factors [3]. Creatinine, hemoglobin and NT-proBNP on admission did not predict CI-AKI occurrence.
4.3.2. Procedure related risk factors
Our study demonstrates that procedure-related factors play a significant role in the development of AKI after CRT implantation. The predictive value of these factors has not been studied before in CRT patients. A larger procedure time, intraoperative hypotension and intraoperative bleeding, showed to significantly predict AKI in CRT patients, as assessed by binary logistic regression models.
4.4. Impact on 1-year mortality
Among the few studies, that investigated the incidence of AKI in patients undergoing CRT implantation, to our best knowledge, only one of them studied the impact on long-term outcomes. A sub-analysis of the TRUST CRT trial, which studied a relatively young group of HF-patients (median of 61 years), revealed a significant impact of AKI occurrence on long-term mortality, with almost three-fold higher 30-month mortality in the AKI group [5]. Our findings on long-term outcomes of elderly patients are in line with those presented by Kowalczyk et al. regarding the higher 12-month hospitalization rate and mortality, however, our results did not reach statistical significance. This lack of statistical significance may be explained by the lower number of patients included in our study and the shorter follow-up period. Future prospective studies are needed to evaluate the negative impact of AKI on long-term mortality.
4.5. Clinical implications of this study
Our findings provide deeper insight and knowledge about the possible mechanisms and preventive strategies of AKI post CRT. Furthermore, our study contributes real-life data in an elderly (>75 years old) CRT-HF population, thus shedding light on a category of patients which is commonly under-represented in CRT trials. Our study suggests that AKI is a frequent complication with an important impact on LOS and long-term mortality. The effort of minimizing this complication should, therefore, hold a high priority when CRT implantation is performed. Some of the procedure-related factors share a common pathophysiologic pathway and could interact with each other. Intra-operative bleeding may lead to hipotension and eventually cause renal hipoperfusion. A longer procedure time may be due to these complications and may also be associated with a higher use of contrast medium. This is underlined by the fact that 2 of 3 procedure-related factors did not maintain statistical significance when included in a multivariate logistic regression model that incorporated all variables that showed to be significant predictors in univariate models. However, prior impairment of the renal function showed predict AKI in all models and consequently seems be an independent risk factor. Thus, a thorough patient selection and the avoidance of factors that could lead to peri-procedural renal hipoperfusion are crucial to reduce the high incidence of AKI.
4.6. Study limitations
The most important limitations of our study are the single centre retrospective design and the insufficient data on total contrast volume used in all patients. Furthermore, the relatively low number of patients is a limiting factor concerning the interpretation of multivariate regression models.
4.7. Conclusions
In conclusion, we found that AKI is a frequent complication of CRT implantation with an important negative influence on in-hospital stay, especially in the elderly. In addition to the administration of contrast medium, patient and procedure-related factors, such as prior renal dysfunction, larger procedure time, intra-operative hypotension and -bleeding, could play a significant role in the occurrence of AKI.
CRediT authorship contribution statement
Alexander Marschall: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing - original draft, Writing - review & editing. Hugo del Castillo Carnevalli: Investigation, Methodology, Resources, Validation, Writing - review & editing. José Carlos de la Flor Merino: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing - review & editing. Miguel Rubio Alonso: Resources, Validation, Writing - review & editing. Ramón de Miguel Gómez: Resources, Validation, Writing - review & editing. Jorge Palazuelos Molinero: Validation, Writing - review & editing. María de Fatima Goncalves Sánchez: Resources, Validation, Writing - review & editing. Edurne López Soberon: Validation, Writing - review & editing. Concepción Fernández Pascual: Validation, Writing - review & editing. Ricardo Concepción Suárez: Validation, Writing - review & editing. Dámaris Carballeira Puentes: Validation, Writing - review & editing. Freddy Andrés Delgado Calva: Validation, Writing - review & editing. Salvador Álvarez Antón: Resources, Validation, Writing - review & editing. David Martí Sánchez: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgments
Acknowledgement
None.
Statement of Ethics
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a priori approval by the institution's human research committee.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
None declared
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100594.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Anon. 2013 ESC Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy. Europace. 2013 Aug;15(8):1070-118. doi: 10.1093/europace/eut206. [DOI] [PubMed]
- 2.Singh J.P., Klein H.U., Huang D.T. Left ventricular lead position and clinical outcome in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT) trial. Circulation. 2011;123:1159–1166. doi: 10.1161/CIRCULATIONAHA.110.000646. [DOI] [PubMed] [Google Scholar]
- 3.Mehran R., Aymong E.D., Nikolsky E. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention. J. Am. Coll. Cardiol. 2004;44:1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 4.Cowburn P.J., Patel H., Pipes R.R. Contrast nephropathy post cardiac resynchronization therapy: an under-recognized complication with important morbidity. Eur. J. Heart Fail. 2005;7:899–903. doi: 10.1016/j.ejheart.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Kowalczyk J., Lenarczyk R., Kowalski O. Triple-site versus standard cardiac resynchronization trial (TRUST CRT) investigators. Contrast-induced acute kidney injury in patients undergoing cardiac resynchronization therapy-incidence and prognostic importance. Sub-analysis of data from randomized TRUST CRT trial. J. Interv. Card. Electrophysiol. 2014;40:1–8. doi: 10.1007/s10840-014-9887-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tester G.A., Noheria A., Carrico H.L. Impact of radiocontrast use during left ventricular pacemaker lead implantation for cardiac resynchronization therapy. Europace. 2012;14:243–248. doi: 10.1093/europace/eur282. [DOI] [PubMed] [Google Scholar]
- 7.Zareba W., Klein H., Cygankiewicz I. MADIT-CRT investigators. effectiveness of cardiac resynchronization therapy by QRS morphology in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT) Circulation. 2011;123:1061–1072. doi: 10.1161/CIRCULATIONAHA.110.960898. [DOI] [PubMed] [Google Scholar]
- 8.Moss A.J., Brown M.W., Cannom D.S. Multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT): design and clinical protocol. Ann Noninvasive Electrocardiol. 2005;10:34–43. doi: 10.1111/j.1542-474X.2005.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neumann FJ, Sousa-Uva M, Ahlsson A et al. [2018 ESC/EACTS Guidelines on myocardial revascularization]. Kardiol Pol 2018;76:1585–1664. [DOI] [PubMed]
- 10.Hallan S., Asberg A., Lindberg M. Validation of the Modification of Diet in Renal Disease formula for estimating GFR with special emphasis on calibration of the serum creatinine assay. Am. J. Kidney Dis. 2004;44:84–93. doi: 10.1053/j.ajkd.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 11.Lasser E.C., Lyon S.G., Berry C.C. Reports on contrast media reactions: analysis of data from reports to the U.S. Food and drug administration. Radiology. 1997;203:605–610. doi: 10.1148/radiology.203.3.9169676. [DOI] [PubMed] [Google Scholar]
- 12.Rihal C.S., Textor S.C., Grill D.E. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 13.McCullough P.A., Wolyn R., Rocher L. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am. J. Med. 1997;103:368–375. doi: 10.1016/s0002-9343(97)00150-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.