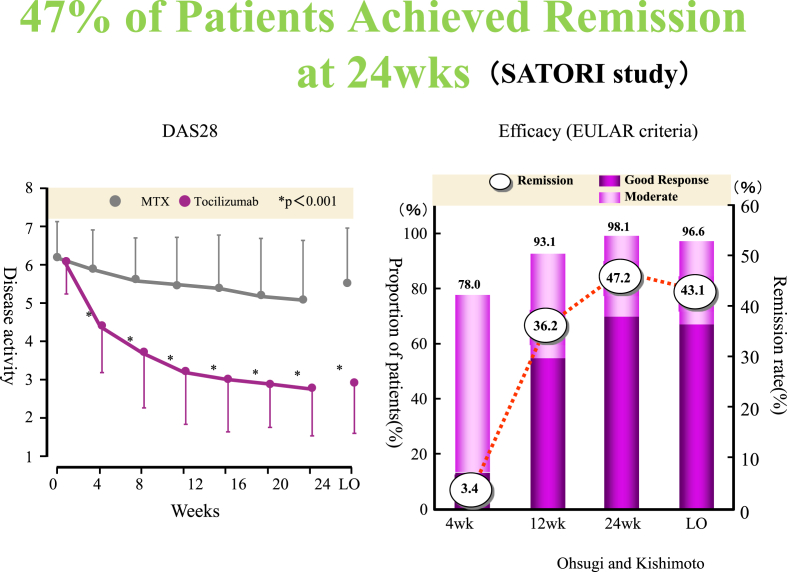
Fig. 7.
Clinical phase III study (named “Satori Study”). Approximately half of the patients had achieved clinical remission by the 6-month evaluation. (Left) The vertical axis is the DAS28 value (disease activity). Data is shown as an average value of all cases and standard error (because it gets complicated, the vertical axis showing the standard error shows only on one side). The horizontal axis shows the time (in weeks) from the time that tocilizumab was administered (1 time every 4 weeks). The DAS28 value is not fluctuating much in the control group, but in the group to which tocilizumab was administered, it is obviously decreasing, showing improved symptoms. In any of the 4-week administration periods, statistically significant improvement can be recognized (marked with a * symbol). (B) The vertical axis on the left side is the percentage (%) of patients that achieved improvement in their symptoms (complete response and effect). The vertical axis on the right side shows (bar graph) the DAS28 remission rate (see Note below). As administration continued, the ratio of patients with improved symptoms increased, and after 24 weeks (after 6 administrations), the ratio of patients who have achieved “Complete Response” or “Effect” reached 98%. The number inside the circle shows the DAS28 remission rate (dotted line polyline graph). After 24 weeks, 47% achieved remission. (Note) DAS28. Used as a standard for showing disease activity level using a score calculated based on a calculation method established by EULAR (the European League of Arthritis and Rheumatism). Sum total of the number of joints showing inflammation or pain amongst 28 fixed joints, and erythrocyte sedimentation rate, and for total symptoms, multiplying their respective values by an established coefficient. If the DAS28 score is below 2.6, it is considered remission. (Adapted from Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J, Kishimoto T. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol. 2009; 19:12–19).