Table 3.
Reductive amination of aromatic ketonesa
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Ketone | Conv. (%)b | 6 (%)b | 7 (%)b | 8 (%)b | |
| 1 | 5a | R = Ph R’ = Me |
>99 | 99 (96c) | <1 | <1 |
| 2 | 5b | R = p-OMePh R’ = Me |
>99 | >99 (93c) | <1 | <1 |
| 3 | 5c | R = p-OHPh R’ = Me |
>99 | >99 | <1 | <1 |
| 4 | 5d | R = p-BrPh R’ = Me |
>99 | 87 (82c) | <1 | 13 |
| 5 | 5c | R = p-FPh R’ = Me |
81 | 79 | <1 | 2 |
| 6 | 5f | R = p-NO2Ph R’ = Me |
>99 | 92 (87c) | <1 | 8 |
| 7 | 5g | R = m-NO2Ph R’ = Me |
>99 | >99 | <1 | <1 |
| 8 | 5h | R = p-CNPh R’ = Me |
>99 | 96 | 4 | <1 |
| 9 | 5i | R = Ph R’ = CF3 |
>99 | 98 | <1 | <1 |
| 10 | 5j |  |
>99 | 99 | <1 | <1 |
| 11 | 5k |  |
86 | 81 | <1 | <1 |
| 12 | 5l |  |
20 | 20 | <1 | <1 |
| 13 | 5m |  |
64 (>99d) | 63 (70c,d) | <1 | <1 |
| 14 | 5n | 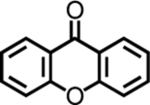 |
<1 | 0 | 0 | 0 |
Reagents and conditions: ketone (100 μmol), Ir1 (1.0 μmol), HCOONH4 (1.0 mmol), CH3OH (0.5 mL), 37 °C, 15 h.
Determined by 1H NMR with 1,3,5-trimethoxybenzene as an internal standard. Each experiment was performed in triplicate and the average yield was calculated.
Isolated yield.
Reaction was run for 24 h.
