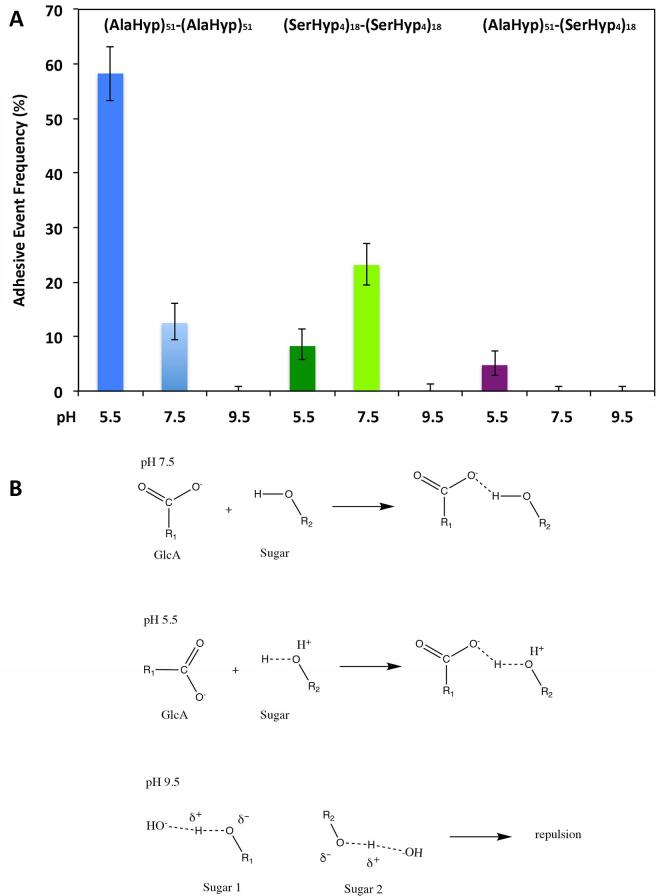
Fig. 5.
Adhesion events recorded during the homomolecular and heteromolecular interactions of the glycomodules at different pH condition (A) and the possible adhesion mechanisms (B). (A) The microcantilevers were coated with 6 (AlaHyp)51 glycomodules per 1000 nm2 or 70 (SerHyp4)18 glycomodules per 1000 nm2. The beads used for (AlaHyp)51 to (AlaHyp)51 glycomodule adhesion at pH 9.5 and 7.5 were coated with 2 (AlaHyp)51 glycomodules/1000 nm2, while with 1 (AlaHyp)51 glycomodule/2000 nm2 for (AlaHyp)51 homomolecular adhesion at pH 5.5. The (SerHyp4)18 glycomodule beads were coated with 30 molecules/1000 nm2. The adhesive event rate was measured as a percentage of adhesive events from hundreds of test cycles. (B) At pH 7.5, H-bonds are formed between GlcA and hydroxyls of other sugars; while at pH 5.5 hydroxyl proton that is loosely attached to hydroxyl oxygen due to the attack of H+ on the hydroxyl oxygen forms stronger H-bond with oxygen of GlcA carbonyl. However, at pH 9.5, the hydroxyl proton is pulled off by −OH, which resulted in a partially negatively charged sugar. The negative charges of different sugars repel each other and prevent intermolecular adhesion.