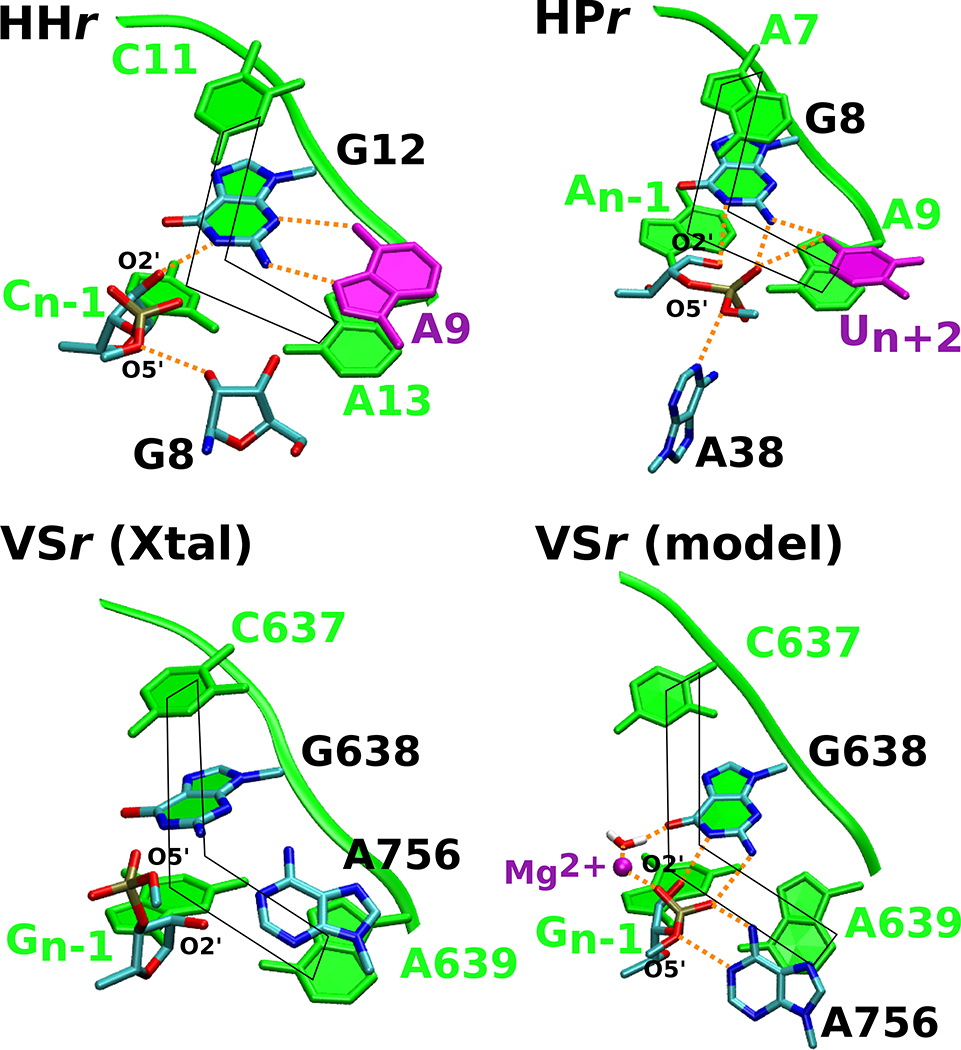
Figure 5. The hammerhead, hairpin and VS ribozymes use distinct strategies to anchor the L-platform.
Active sites in the crystal structures of the three ribozymes are compared to the Mg2+-bound VS ribozyme model proposed in this work to highlight common features. Each of the ribozyme active sites exhibit the L-platform motif (shown in fade green) formed by the N-1 nucleotide and nucleotide 3’ to the general base as the base of the L, and the nucleotide 3’ to the general base, general base itself, and the nucleotide 5’ to the general base as the long arm of the L. Both the hammerhead and hairpin contain a L-anchor residue (shown in purple) that engages with the general base and presumably holds it in position; this active site feature is lacking in the VS ribozyme crystal structures. The Mg2+-bound model defined in this study suggests that the metal ion steps into the role of the L-anchor, interacting with G638 and positioning the base for catalysis. In this figure, the hairpin, hammerhead, and VS ribozyme active sites are based on PDB IDs 1M5O,50 3ZP8,51 and 5V3I,20 respectively.