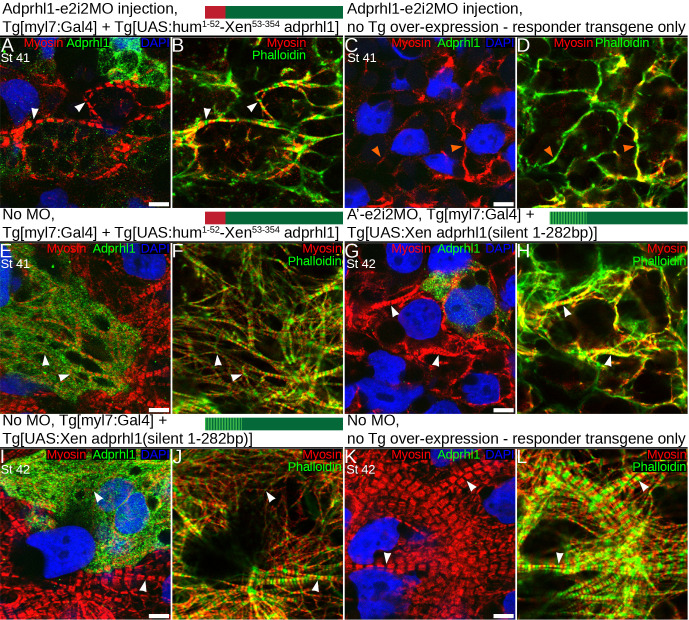
Fig 2. Limited recovery of cardiac myofibril assembly in adprhl1 morpholino injected embryos by transgenic synthesis of recombinant 40 kDa Adprhl1 proteins.
Experiments that combine adprhl1 MO knockdown with two distinct transgenes engineered to achieve adprhl1 over-expression. This is the concise version of S2 Fig. For brevity, Fig 2 presents only the high magnification (D and E) images that reveal ventricle wall myofibril patterns within the experimental hearts. The extended figure additionally includes the morphology of each ventricle, the extent of Adprhl1 protein production within, plus squares to locate the position of the myofibril images within each ventricle. A, B: Cardiomyocytes within the heart ventricle wall (anterior surface) of a stage 41 tadpole that was injected with the RNA-splice interfering MO, Adprhl1-e2i2MO, into dorsal (D-2/4) blastomeres. Additionally, it carried binary transgenes to over-express recombinant Adprhl1 protein, consisting of Tg[myl7:Gal4] driver and the Tg[UAS:human1-52-Xenopus53-354 adprhl1] responder. Scale bar = 5 μm (all panels). Fluorescence image (A) shows anti-Adprhl1 immunocytochemistry (green), anti-myosin (red) and DAPI-stained nuclei (blue). The second panel (B) displays a merge of myosin and phalloidin actin stain, with the phalloidin coloured green to evaluate signal overlap. C, D: Ventricle cardiomyocytes from a sibling tadpole that received the same Adprhl1-e2i2MO injection but carried only the UAS-responder transgene and hence did not produce excess recombinant Adprhl1. E, F: A double transgenic sibling that synthesized recombinant human-Xenopus hybrid Adprhl1 but was not injected with the MO. G, H: Ventricle cardiomyocytes from a second experiment, a stage 42 tadpole that was injected with Adprhl1-e2i2MO and carried the Tg[myl7:Gal4] driver but a different Tg[UAS:Xenopus adprhl1(silent 1-282bp)] responder transgene. This incorporates silent nucleotide changes (synonymous substitutions) to the cDNA sequence in order to partially evade endogenous translational regulation. I, J: Ventricle cardiomyocytes of a double transgenic, silent mutation, sibling tadpole that synthesized recombinant Xenopus Adprhl1 but was not injected with the MO. K, L: A non-injected sibling control harbouring only the silent mutation responder transgene that did not produce excess recombinant Adprhl1. Paired white arrowheads indicate Z-disc sarcomere positions, orange arrowheads denote non-striated filaments. V, ventricle; OT, outflow tract.