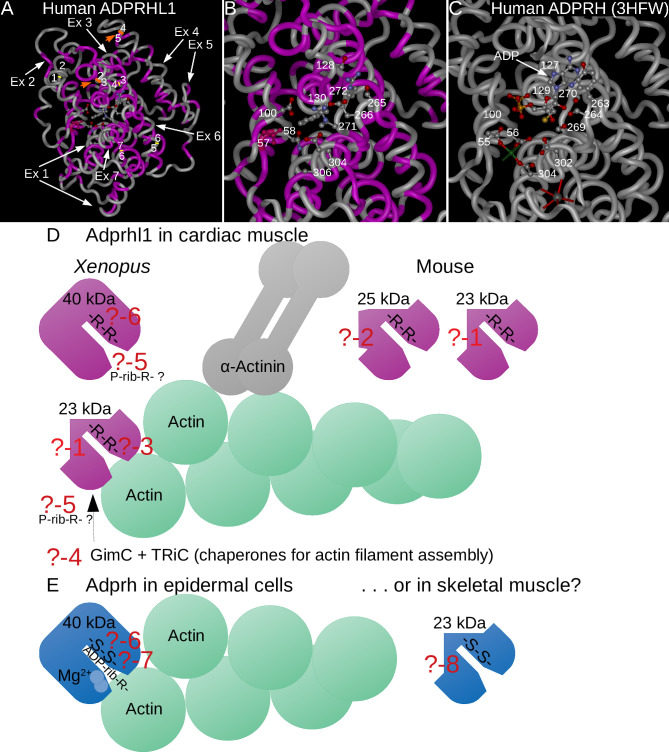
Fig 9. Questions regarding Adprhl1 (and Adprh) action.
A-C: Structural model of human ADPRHL1 with protein backbone drawn as a tube (A) and magnified active site region (B) [10] that is based on the solved crystal structure of human ADPRH (3HFW) (C-active site shown only) [43]. Amino acids that are common to both proteins are coloured magenta (A, B). White arrows indicate the contribution made by each ADPRHL1 exon to the sequence, orange arrows highlight the positions encoded by the exon-2-3 and exon-4-5 boundaries, with yellow lines marking the remaining exon borders (A). The translated exon-2-3 and 4–5 boundaries reside close to each other and have parallel alignment. Thus a smaller 23 kDa ADPRHL1 form that lacks aa sequence from exons 3–4 could conceivably retain a similar protein fold. Select aa side chains within the active sites are shown as ball and sticks models. For active enzyme ADPRH, aspartates-55, 56, 302, 304 (Mg2+ coordination and catalysis), Cys129, Tyr263, serines-264, 269, 270 (substrate binding cleft) and the location of glycines-100, 127 are shown (C). A molecule of ADP occupies the active site, along with one of the two Mg2+ cations (green sphere) and a K+ ion (grey sphere) (C). For cardiac ADPRHL1, the corresponding active site residues are mostly changed. Asp57 (conserved and coloured red), Asn58, Glu302, Ala304 (no cation coordination), Phe130, Ser265, Glu266, arginines-271, 272 (changed substrate cleft), plus Asp100, Ser128 are shown (B). With this altered active site, ADP cannot be forcibly docked into the ADPRHL1 model. D, E: A deeper understanding of Adprhl1 action is required in order to describe how myofibrillogenesis is linked to chamber outgrowth in the embryo. This diagram illustrates several questions that will need to be addressed:? -1: What is the precise composition of the smaller 23 kDa Adprhl1 protein? The mouse em1 allele has already provided some information. It does not contain exons 3–4 encoded sequence but will include exons 5–6 sequence recognised by the peptide antibody.? -2: What is the composition of the additional 25 kDa mouse Adprhl1 protein? It is likely to be related to the 23 kDa species.? -3: Which sequences localize Adprhl1 to Z-disc/actin filament barbed end boundaries and what myofibril components does it associate directly with? The localization is observed when using an N-terminal epitope tag but not with the exons 5-6-specific antibody. The epitope for the peptide antibody is possibly obscured when Adprhl1 associates with myofibrils.? -4: Might Adprhl1 cooperate with the chaperones and co-chaperones that fold and assemble actin filaments? GimC and TRiC are among factors known to contribute to actin dynamics. There are many additional components of the functional sarcomere unit of myofibrils that Adprhl1 could interact with.? -5: Does Adprhl1 retain binding activity for a post-translational modification that is related to ADP-ribose? For example, an ADP-ribose modification can be partially degraded to a smaller phospho-ribose group by a pyrophosphatase or phosphodiesterase reaction.? -6: Could the substrate binding clefts of Adprhl1 and Adprh also be targets for ADP-ribosylation? Comparison of the di-arginine versus di-serine residues present in the active sites of the two proteins. Di-arginine is common among verified sites for ADP-ribosylation on arginine side chains. Moreover, serine is another acceptor site for ADP-ribosylation that can be hydrolysed by the action of Adprhl2.? -7: Does the active enzyme Adprh contain sequences that provide specificity for particular target proteins, in addition to the binding and hydrolysis of ADP-ribosylated arginine? In the illustration, Adprh is depicted acting on ADP-ribosylated actin, one of a number of known (arginine acceptor) targets of bacterial toxin ADP-ribosyltransferases. Could domains adjacent to the active site help stabilize the interaction with particular modified target proteins. Might this be the activity that is common to both Adprh and Adprhl1.? -8: Could smaller protein forms of Adprh also exist, for example in skeletal muscle? There could yet be analogy between 23 kDa Adprhl1 action in cardiac muscle and Adprh in skeletal muscle. However, evidence against this comparison would be that exon structure is not conserved between the two family members.