Fig. 2.
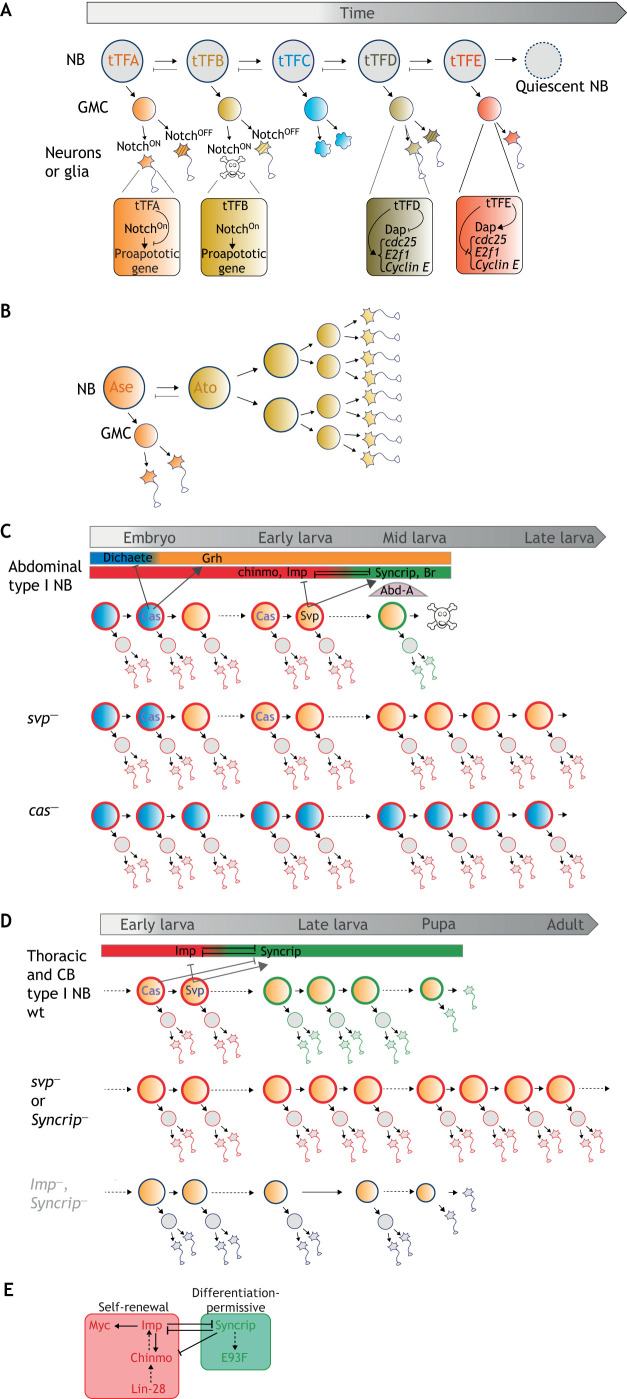
Stereotyped regulation of neural progenitor proliferation by temporal patterning. (A) Temporal regulation of daughter cell divisions or apoptosis. Notch signaling in GMCs gives rise to binary cell fate decisions in the two post-mitotic progeny. The NotchON state can trigger activation of pro-apoptotic genes leading to the elimination of one of the progeny. Some tTFs have the ability to repress NotchON-mediated apoptosis. tTFs can also block the division of GMCs or promote NB quiescence by activating Dap and silencing cell-cycle genes (string/cdc25, E2f1, Cyclin E), leading to their terminal differentiation without division. (B) Temporal regulation of an asymmetric-to-symmetric division switch. tTF progression can also promote a switch in the NB mode of division. In the larval optic lobe, a subset of NBs undergoes an asymmetric-to-symmetric division switch triggered by the sequential expression of Ase and Ato. (C) Temporal regulation of NB apoptosis. Permanent transcriptional switches induced by tTFs (embryonic expression of cas and larval expression of svp) schedule the competence for NBs to undergo apoptosis upon expression of abd-A. (D) Temporal regulation of NB self-renewal. Sequential expression of cas and svp promotes the Imp-to-Syncrip switch during mid-larval stages. NBs that fail to undergo the Imp-to-Syncrip switch do not stop dividing during pupal stages and continue dividing in adults. NBs lacking both Imp and Syncrip exhibit a lower growth and proliferative potential. (E) Imp, together with Chinmo, Lin-28 and Myc, compose a self-renewing module in early larval NBs. Syncrip and E93 are part of an antagonistic module that induces a differentiation-permissive state in NBs and exhausts their self-renewing potential. CB, central brain; wt, wild type.