Fig. 1.
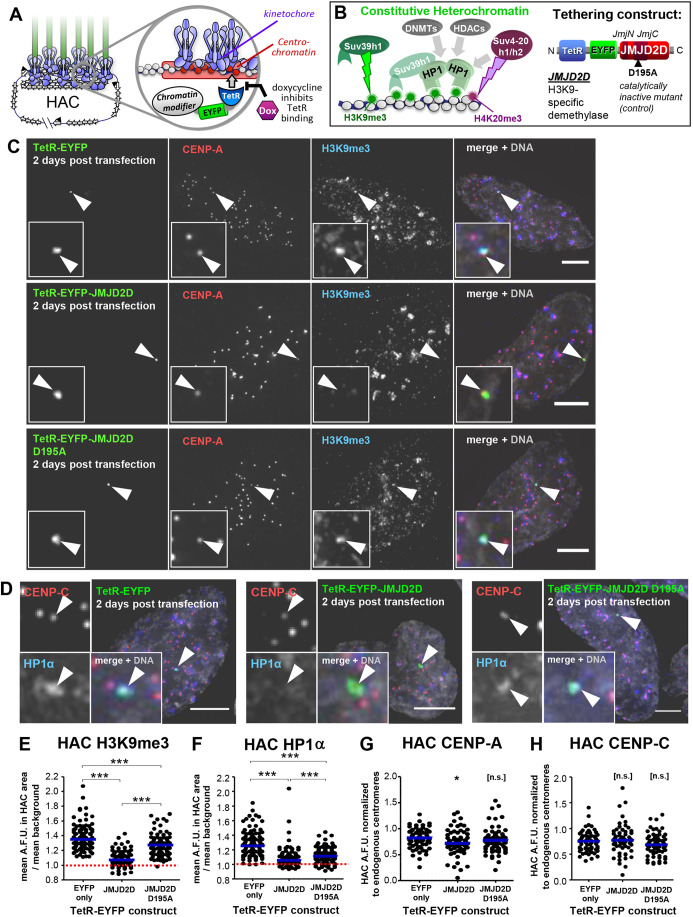
JMJD2D efficiently removes heterochromatin from the HAC. (A) Schematic representation of HAC structure and control of TetR-fusion-protein tethering by doxycycline. (B) Simplified representation of the constitutive heterochromatin recruitment pathway and the TetR–EYFP–JMJD2D fusion protein used to remove H3K9me3 from the HAC. (C) JMJD2D tethering specifically removes H3K9me3 from the HAC. Immunofluorescence analysis of interphase HeLa-HAC-2-4 cells, 48 h after transient transfection with plasmids expressing TetR–EYFP, TetR–EYFP–JMJD2D and TetR–EYFP–JMJD2DD195A. Arrowheads locate the HAC. Scale bars: 5 μm. (D) JMJD2D tethering delocalizes HP1 from the HAC. Experimental details as in C. Scale bars: 5 µm. (E,F) JMJD2D tethering efficiently and specifically removes H3K9me3 from the HAC, and delocalizes HP1α. Quantification of mean HAC-associated H3K9me3 or HP1α immunofluorescence signal. Median is shown with blue bars; red dashed line indicates mean nuclear background level. H3K9me3, total of three biological repeats, n=13–46 cells each; HP1α, total of three biological repeats, n=22–45 transfected cells each. (G,H) JMJD2D tethering to the HAC for 2 days has little effect on CENP-A and CENP-C. Quantification of HAC-associated immunofluorescent signal. CENP-A, total of two biological repeats, n=27–36 transfected cells each; CENP-C, total of two biological repeats, n=26–34 cells each. Median is shown with blue bars. *P<0.05; ***P<0.0005; n.s., not significant (Mann–Whitney U test).