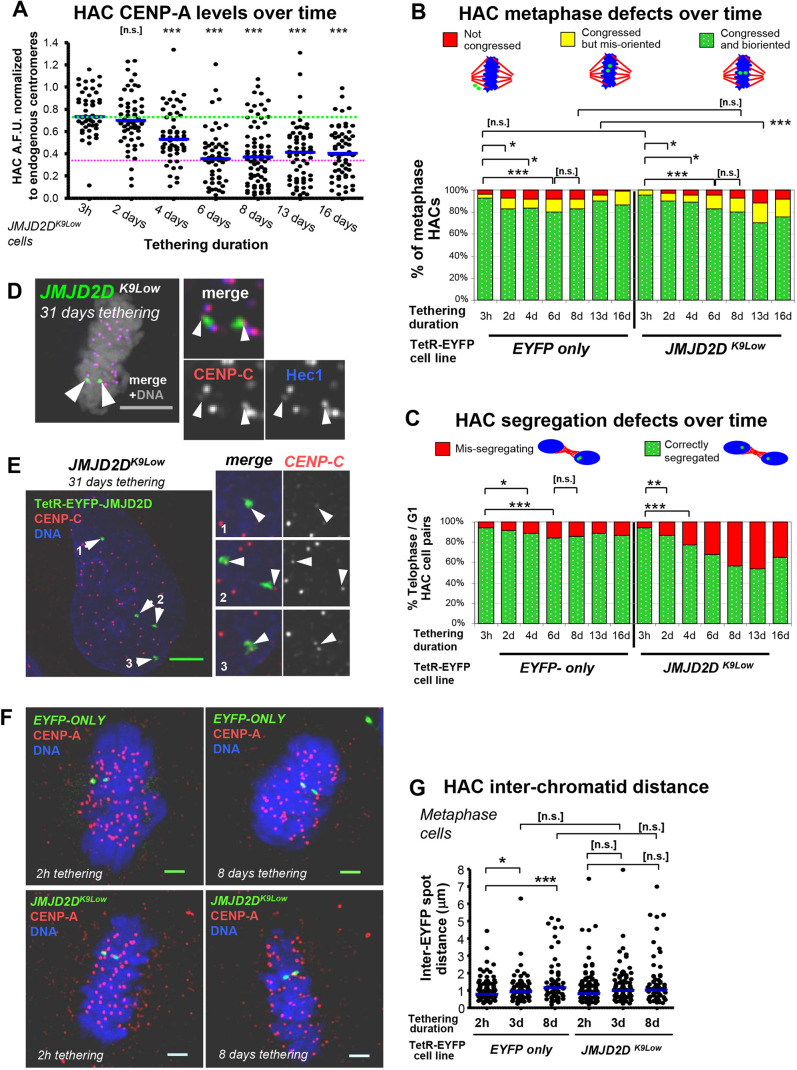
Fig. 6.
Long-term JMJD2D tethering induces progressive reduction of HAC CCAN and increase in mis-segregation, but HAC centromere is not abolished. (A) Time-course of long-term JMJD2D tethering indicates that the HAC centromere is not abolished. JMJD2DK9Low cells were washed of doxycycline and grown for several days, and samples were taken in intervals. The mean HAC-associated CENP-A immunofluorescence signal was measured, and normalized to that of endogenous centromeres. Total of two biological replicates, n≥22 interphase cells each. Blue bar indicates median, green dotted line indicates median CENP-A levels at the start of the time-course, magenta dotted line indicates 32.9% of the median endogenous CENP-A level. ***P<0.0005; n.s., not significant (Mann–Whitney U test). (B,C) JMJD2D tethering causes few observable metaphase defects, but HAC mis-segregation increases progressively until ∼8 days, but no further. Time-course analysis as described in A, but examining HAC metaphase phenotypes, or mis-segregation defects in telophase or early G1 cells. Sum of two biological repeats, n≥80 cells (metaphase), n≥100 cells (segregation) each. *P<0.05; **P<0.005; ***P<0.0005; n.s., not significant (Fisher's exact test). (D,E) HAC centromere persists even upon very long tethering durations and retains ability to congress and bi-orient on the metaphase plate. Reduced but still present signals for CENP-C can be observed on HAC interphase centromeres, and also Hec1 in metaphase chromatids, by immunofluorescence. Arrowheads locate the HAC. Scale bars: 5 μm. (F,G) Images and quantification showing that HAC sister chromatid cohesion during metaphase is not significantly affected by short or long-term JMJD2D tethering, and subsequent H3K9me3 removal. Interchromatid distance was measured between the two HAC EYFP fluorescent signals, in fixed metaphase cells. Scale bars: 2 μm. *P<0.05; ***P<0.0005; n.s., not significant (Mann–Whitney U test.).