Table 1.
Synthetic scope of alcohols in enantioselective, radical C–H amination.
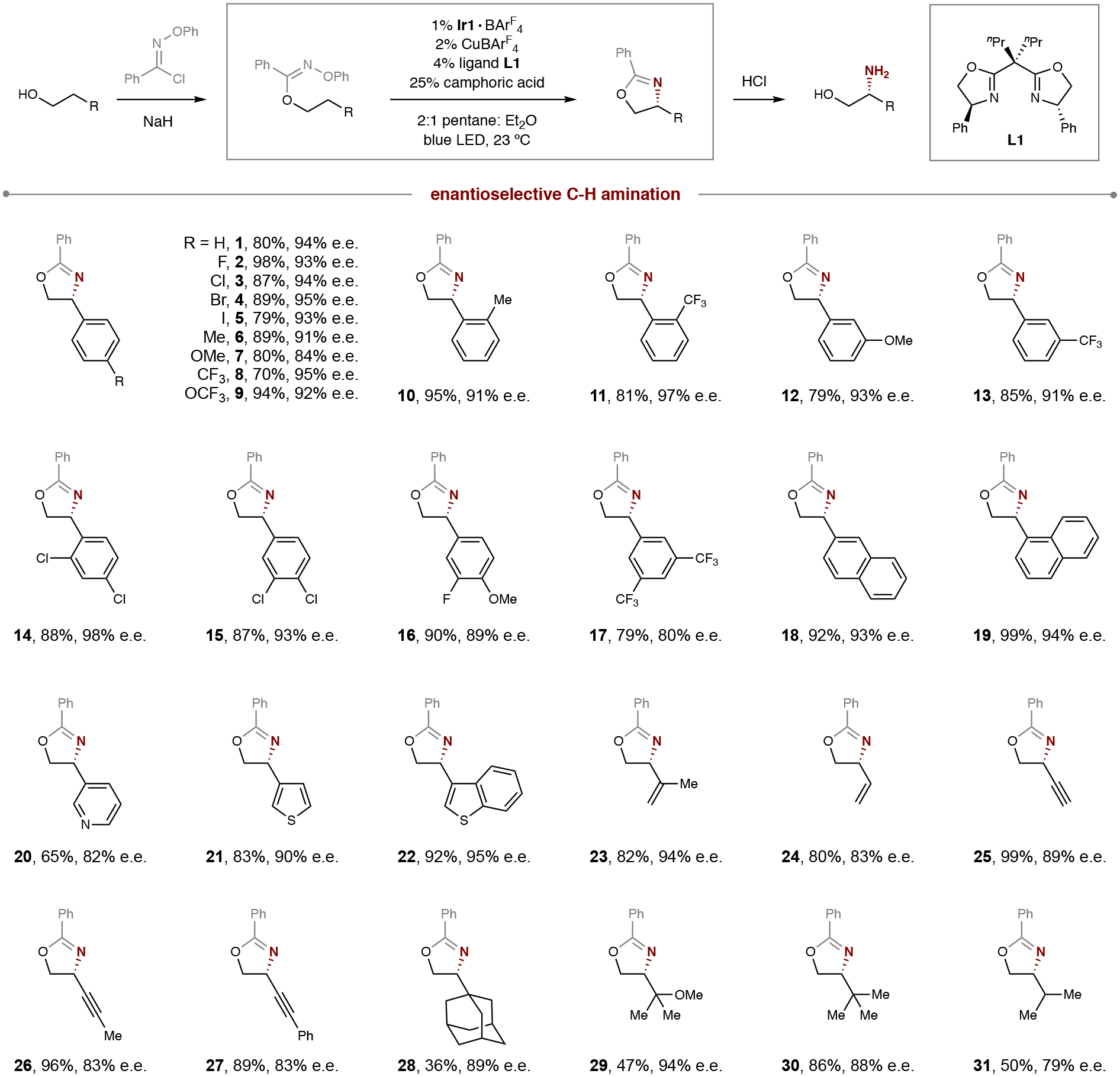
|
Conditions: 1% Ir{[dF(CF3)ppy]2dtbbpy}BArF4, 2% CuBArF4, 4% ligand L1, 25% camphoric acid, pentane: Et2O (2:1), blue LED irradiation, room temperature, 1 hour. See General Procedure 3 in SI section II for full experimental details. Isolated yield and enantiomeric excess (e.e.) indicated below each entry.
