Table 2.
Synthetic scope and effect of chaperone in enantioselective, radical C–H amination.
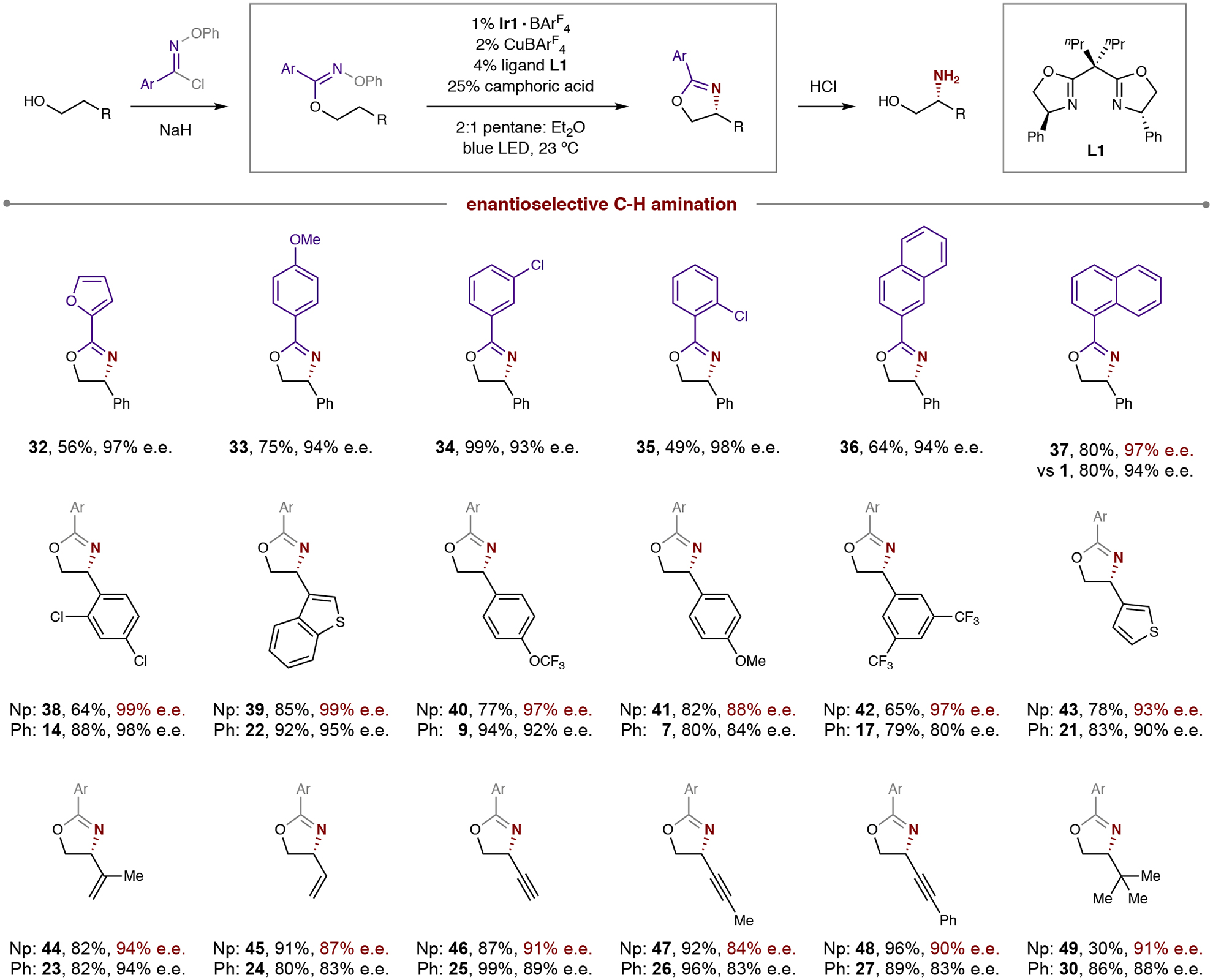
|
Conditions: 1% Ir{[dF(CF3)ppy]2dtbbpy}BArF4, 2% CuBArF4, 4% ligand L1, 25% camphoric acid, pentane:Et2O (2:1), blue LED irradiation, room temperature, 1 hour. See General Procedure 3 in SI section II for full experimental details. Isolated yield and enantiomeric excess (e.e.) indicated below each entry. Np affords improved enantioselectivity in many cases. Abbreviations: Ar, aryl, Np, 1-naphthyl.
