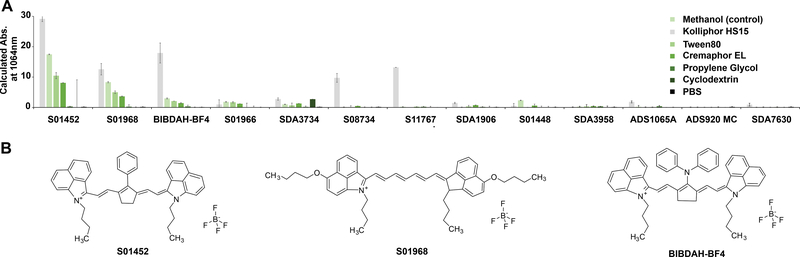
Fig 1. Screening NIR-II dyes with simple formulation in solubilization systems suitable for intravenous delivery.
A) Calculated absorption at 1064 nm of indicated dyes after formulation in the indicated solvent or surfactant systems. The final concentration of the indicated solubilization agent was 10 % (v/v or v/w) in phosphate buffered saline (PBS), with the exception of propylene glycol, which had a final concentration was 40% with 10% ethanol. Methanol is a control solvent that is not a suitable carrier for intravenous administration. Error bars show std. dev., n=3. B) Chemical structures of the indicated dyes.