Figure 2. Ubiquitylation-medicated protein degradation.
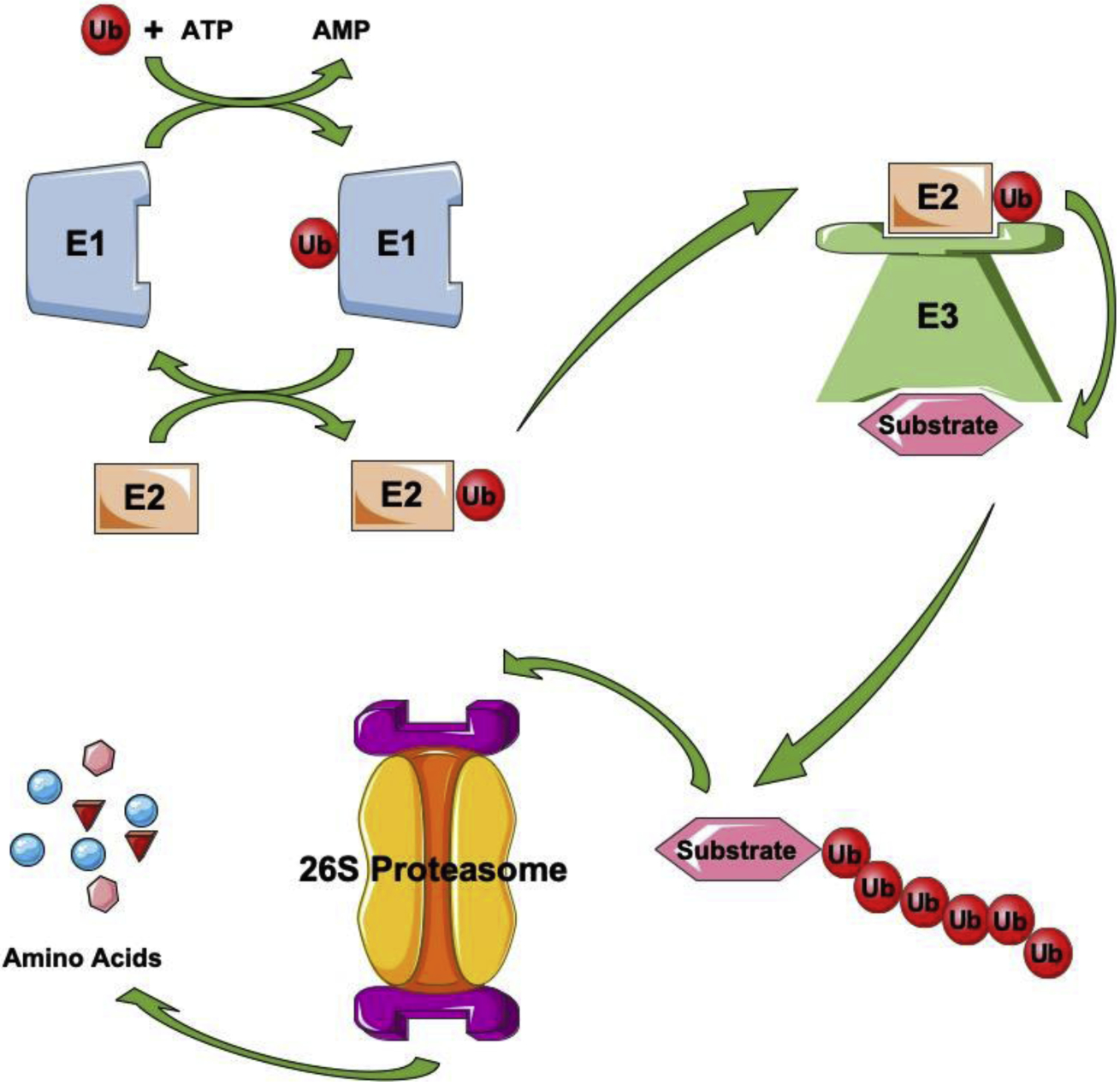
Protein ubiquitylation is catalyzed by the concerted and coordinated action of three enzymes: E1, ubiquitin-activating enzyme that produces an active ubiquitin using energy to generate a thioester linkage between itself and ubiquitin molecule; E2, ubiquitin-conjugating enzyme that is both an ubiquitin receptor for E1 and an ubiquitin donor for substrates or E3s; E3, ubiquitin ligase that binds to E2s and its substrates to assist ubiquitin transfer from E2s to substrates or directly recognizes and conjugates ubiquitin to its targets. Polyubiquitylated proteins will be degraded in proteasome, a complex intracellular structure composed of multiple enzymatic complexes.
