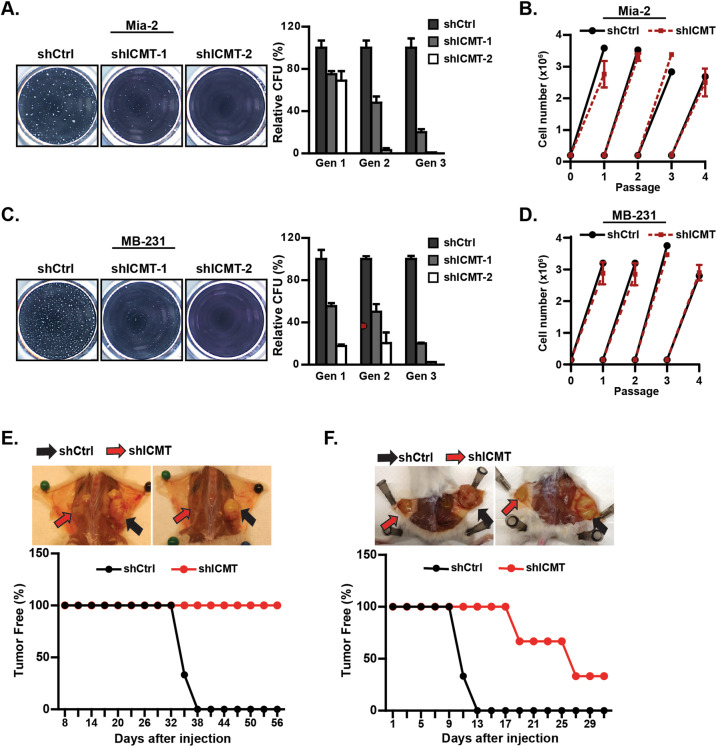
Fig. 1. Suppression of ICMT abolishes the self-renewal ability of mutant KRAS-driven pancreatic and breast cancer cells.
a, c Sphere formation study. MiaPaCa2 pancreatic (a) and MDA-MB231 breast (c) cancer cells were infected with lentivirus expressing either control shRNA or two ICMT-targeting shRNAs, followed by serial replating sphere formation studies. The images of the spheres were obtained after the third plating growth (3rd generation spheres, left panel). The sphere counts from three technical repeats for each replating (generation 1, 2, and 3) were analyzed by OpenCFU and Prism5, and presented as bar graphs (right panels). Each sphere formation assay was performed with three technical repeats; and the experiments were repeated in three biological repeats with similar results. ICMT expression levels, assessed by RT-qPCR in the two cell lines with and without ICMT knockdown, are shown in Supplementary Fig. S1A. Proliferation rates under normal adherent growth conditions of MiaPaca2 (b) and MDAMB-231 (d) cells expressing either control or ICMT shRNA. Shown are the changes in total cell numbers during 3 days of culturing for each passage vs. the passage number, with 3-day intervals between each passage. e, f In vivo tumor formation study. MiaPaca2 (e) and MDA-MB231 (f) cells (80,000 each) expressing either control or ICMT-targeting shRNA were injected subcutaneously into NOD-SCID mice (n = 10 tumors for each group). The mice were observed every 2 days until the largest tumor reach the volume limit set by the IACUC protocol, at which time all the mice were euthanized. The percentages of tumor-free mice were plotted (bottom panels) up to 56 days and 30 days for the tumors derived from MiaPaCa2 (e) and MDA-MB231 (f) cells, respectively.