Table 1.
Selected Optimization Studies
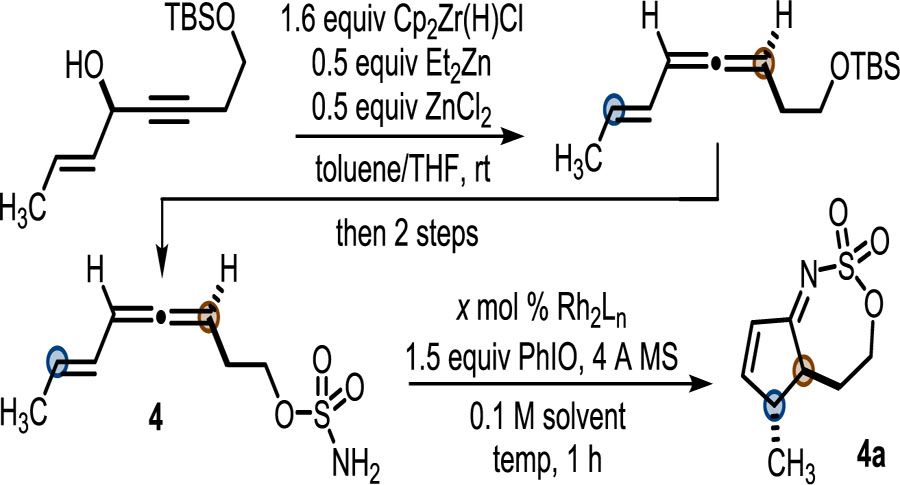 | |||||
|---|---|---|---|---|---|
| entry | solvent | catalyst (mol %) | temp (°C) | yield (%)a | notes |
| 1 | CH2CI2 | Rh2(TPA)4 (1) | rt | 24 | 15% rsm |
| 2 | CH2Cl2 (0.2 M) | Rh2(TPA)4 (1) | rt | 28 | – |
| 3 | CH2Cl2 (0.05 M) | Rh2(TPA)4 (1) | rt | 33 | – |
| 4 | CH2Q2 | Rh2(TPA)4 (1) | 50 | 41 | – |
| 5 | CH2Q2 | Rh2(TPA)4 (1) | 50 | 33 | catalyst added last |
| 6 | CH2Q2 | Rh2(TPA)4 (1) | 50 | 21 | PhI(OAc)2 |
| 7 | MeNO2 | Rh2(TPA)4 (1) | 45 | 23 | – |
| 8 | CH2CI2 | Rh2(TPA)4 (5) | 50 | 55 | LUMO = −2.90 eV |
| 9 | CH2CI2 | Rh2(TPA)4 (5) | 50 | 58 | 0.05 equiv of PhIO × 3, added every 20 min |
| 10 | CICH2CH2CI | Rh2(TPA)4 (5) | 80 | 49 | – |
| 11 | CH2CI2 | Rh2(OAc)4 (5) | 50 | 42 | LUMO = −2.68 eV |
| 12 | CH2CI2 | Rh2(esp)2 (5) | 50 | 41 | LUMO = −2.71 eV |
NMR yields using mesitylene as an internal standard.
