Abstract
Background
Thrombelastography has become increasingly used in liver transplantation. The implications of thrombelastography at various stages of liver transplantation, however, remain poorly understood. Our goal was to examine thrombelastography-based coagulopathy profiles in liver transplantation and determine whether preoperative thrombelastography is predictive of transfusion requirements perioperatively.
Methods
A retrospective review of 364 liver transplantations from January 2013 to May 2017 at a single institution was performed. Patients were categorized as hypocoagulable or nonhypocoagulable based on their preoperative thrombelastography profile. The primary outcome was intraoperative transfusion requirements.
Results
Of patients undergoing liver transplantation, 47% (n = 170) were hypocoagulable and 53% (n = 194) were nonhypocoagulable preoperatively. Hypocoagulable patients had higher transfusion requirements compared to nonhypocoagulable patients, requiring more units of packed red blood cells (7 vs 4, P < .01), fresh frozen plasma (14 vs 8, P < .01), cryoprecipitate (2 vs 1, P < .01), platelets (3 vs 2, P < .01), and cell saver (3 vs 2 L, P < .01). Additionally, these patients were more likely to receive platelets and cryoprecipitate in the first 24 hours following liver transplantation (both P < .05). No differences were found between rates of intensive care unit length of stay, 30-day readmission, or mortality.
Conclusion
Coagulation abnormalities are common among liver transplantation patients and can be identified using thrombelastography. Identification of a patient's coagulation state preoperatively aids in guiding transfusion during liver transplantation. This work serves to better direct clinicians during major surgery to improve perioperative resource utilization. Future prospective work should aim to identify specific thrombelastography values that may predict transfusion requirements.
1. INTRODUCTION
Patients with end-stage liver disease undergoing liver transplantation (LT) often suffer from severe coagulopathy, thrombocytopenia, and platelet dysfunction which place them at increased risk for hemorrhagic complications [1,2]. This clinical dilemma is compounded by the modern understanding that patients with liver disease exhibit an imbalance of both procoagulant and anticoagulant clotting factors [3]. The resulting fragile equilibrium within the coagulation cascade places patients at risk for both bleeding and thrombotic events wherein minor physiological insults can incite hemostatic pathology. Therefore, it is necessary to understand the dynamic coagulation states of these patients perioperatively as they undergo the major physiological stress associated with LT.
Several studies have demonstrated that conventional coagulation tests including prothrombin time, activated partial thromboplastin time, and international normalized ratio (INR) fail to accurately predict bleeding risk in cirrhotic patients [[4], [5], [6], [7], [8]]. In these patients, conventional coagulation tests typically produce a falsely elevated result because they only capture the decreased prothrombotic clotting factors and do not necessarily represent the patient's complex hemostatic state [1,2,9]. Additionally, conventional tests provide a single numerical value which correlates to the time for initial clot formation and lack the ability to describe the dynamic kinetics of the in vivo clotting cascade. As a result, blood product utilization and transfusion requirements during LT remain highly variable and difficult to predict [10].
In contrast, thrombelastography (TEG) is a point-of-care hemostasis assessment tool that offers a more comprehensive evaluation of a patient's coagulation status through direct measurement of the strength of clot formation, stability, and lysis [4,5,10,11]. The hemodilution, acidosis, metabolic byproducts, and hypothermia that occur during LT, particularly during the anhepatic and neohepatic/reperfusion phases, can precipitate coagulopathy [8]. TEG may inform clinicians about the overall coagulation status while factoring in complex hemostatic changes that are occurring at the patient level. Although TEG use has grown within the field of liver transplantation, peri- and intraoperative TEG profiles and the implications of TEG use remain largely unknown. Our aim was to assess the prevalence of TEG-based coagulopathy in patients undergoing LT and determine the significance of a preoperative hypocoagulable state on various intraoperative and perioperative outcomes. We hypothesized that patients would demonstrate major shifts in coagulation profiles during LT and that hypocoagulable patients would have greater blood product transfusion requirements during and after LT.
2. METHODS
2.1. Study population
This study was approved by the University of Cincinnati Institutional Review Board. A retrospective review of a prospectively-maintained LT database was performed to identify all patients undergoing LT between January 2013 to May 2017 at a single academic institution (n = 380). Although our institution does not currently have a formalized TEG utilization protocol for liver transplantation, nearly all patients received a preoperative TEG to establish a baseline for comparison to a postoperative TEG obtained in the intensive care unit (ICU). With increasing TEG use among many surgical specialties, it became increasingly used by our surgical and anesthesia teams intraoperatively to monitor changes in coagulation status along with traditional laboratory assessments. The decision to transfuse was multifactorial based on the clinical situation, judgment of the surgeon and anesthesiologist, and assessment of hemodynamic and physiologic laboratory values. If transfusion was deemed necessary and TEG was available, this coagulation testing guided selection of blood products administered. However, there are no absolute numerical cutoffs for which we transfuse specific blood products empirically based on the TEG measurements.
Available TEG data were reviewed, and only patients with preoperative TEG data were included in this study (n = 364). Subgroup analyses were performed to (1) compare TEG profiles before and after LT for patients who had both preoperative and postoperative data (n = 243) and (2) to evaluate shifts in coagulation profiles during each of the phases of LT (preoperative, anhepatic, neohepatic, postoperative) for those patients with TEG values at each phase (n = 110). Differences between groups were analyzed for demographic data, severity and etiology of end-stage liver disease, donor characteristics, and intraoperative and perioperative transfusion of packed red blood cells (pRBCs), fresh frozen plasma (FFP), platelets, and cryoprecipitate. LT recipient outcomes including durations of intensive care unit (ICU) and hospital stay, and mortality were assessed.
2.2. Thrombelastography classification
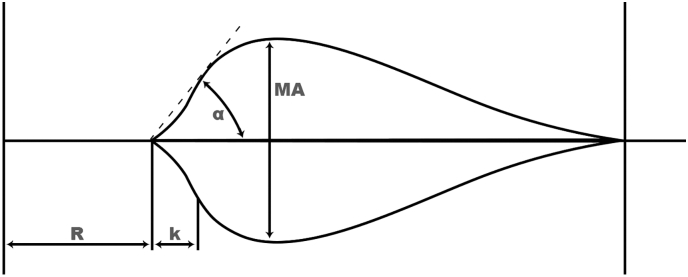
TEG measurements collected for analysis included reaction (R) time, coagulation (k) time, α angle, and maximum amplitude (MA) (Fig. 1). These values were collected at 4 phases of LT: preoperative, anhepatic, neohepatic, and the initial postoperative phase of LT. The preoperative and postoperative TEG readings were collected within 4 hours of the case's start and end times, respectively. Using methodology described by Kaufmann et al, coagulation profiles were categorized as hypocoagulable, hypercoagulable, or normal based on composite rapid TEG profiles of R time, k time, α angle, and MA [12]. Hypocoagulable was defined as having 2 of the following: R time > 44 seconds or k time > 138 seconds, α angle < 64°, and MA < 52 mm. Conversely, hypercoagulable was defined as having 2 of the following: R time < 22 seconds or k time < 138 seconds, α angle > 80°, and MA > 71 mm. The reference ranges (R time 22–44 seconds; k time 34–138 seconds; α angle 64°–80°; MA 52–71 mm) based on laboratory standards and daily quality control samples were used to determine numerical cutoffs of coagulation status.
Fig. 1.
Depiction of a thrombelastography tracing. The x-axis is time and the y-axis is clot strength. R represents time to initiation of clot formation. k and related α angle represent the rate of clot strengthening. MA represents maximal strength of the formed clot.
2.3. Statistical analysis
All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). Given the limited number of hypercoagulable patients, a statistical analysis was performed comparing patients with data for all 4 phases of LT with preoperatively normal (n = 52) and hypercoagulable (n = 4) TEGs. This demonstrated that these patients were not significantly different in demographics or outcomes (data not shown). Therefore, these patients were grouped together as nonhypocoaguable and compared to hypocoaguable patients for this analysis. Continuous data are reported as median and interquartile range (IQR) and compared using the Wilcoxon rank-sum and Kruskal-Wallis tests. Categorical data are reported as total (n) and percentage (%) and compared using the χ2 test (or Fisher exact tests for rare occurrences). Multivariate logistic regression analyses were performed to identify predictors of transfusion requirements within 24 hours of LT. Covariates included preoperative factors of age, sex, ethnicity, body mass index (BMI), coagulation status, Model for End-Stage Liver Disease (MELD) score, preoperative dialysis dependency, admitted at time of LT, and total ischemia time. Statistical significance was set at P < .05.
3. RESULTS
3.1. Study cohort and TEG profiles
The study cohort consisted of 364 patients with complete TEG data who underwent LT during the study period (Table 1). Among all patients, 170 (46.7%) presented for LT in a hypocoagulable state. Among those who had both a pre- and post-LT TEG, 39.9% (n = 97) finished the operation in a different category of coagulation from which they started (Table 2). A total of 44.6% (n = 54) were hypocoagulable to begin the LT but returned to the ICU nonhypocoagulable, whereas 35.3% (n = 43) were nonhypocoagulable and arrived to the ICU hypocoagulable. Next, a subgroup analysis of patients with complete TEG data for all phases of LT (n = 110) was performed. During LT, there was a notable increase in hypocoagulability as the operation progressed (Table 3). Although 49.1% (n = 54) of patients started the operation in a hypocoagulable state, this number increased to 66.4% (n = 73) in the anhepatic phase and 75.5% (n = 83) in the neohepatic phase. This trend toward becoming hypocoagulable was largely resolved by the end of the operation, as 45.5% (n = 50) of patients demonstrated a postoperative hypocoagulable TEG.
Table 1.
Cohort demographics
| Liver transplants (n = 364) N (%)/Median (IQR) |
||
|---|---|---|
| Age, y | 58 | (51–63) |
| Sex, male | 231 | (63.5%) |
| BMI, kg/m2 | 30 | (25–34) |
| MELD (allocation) | 23 | (21–28) |
| Ethnicity | ||
| White | 327 | (89.8%) |
| African American | 33 | (9.1%) |
| Other | 4 | (1.1%) |
| Etiology | ||
| ETOH | 77 | (21.2%) |
| NASH | 83 | (22.8%) |
| HBV/HCV | 110 | (30.2%) |
| Other | 94 | (25.8%) |
| Dialysis prior to LT | 58 | (15.9%) |
| LT type | ||
| Whole | 331 | (91.2%) |
| SLK | 32 | (8.8%) |
| Donor age, y | 38 | (27–52) |
| Donor BMI, kg/m2 | 28 | (24–32) |
| DCD | 24 | (6.6%) |
| Total ischemia time, min | 352 | (290–445) |
| Biliary complications | 65 | (17.86%) |
| Hepatic artery thrombosis | 10 | (2.7%) |
| Primary nonfunction | 3 | (0.8%) |
| Acute cellular rejection | 12 | (3.3%) |
| Length of stay, d | 8 | (6–16) |
| 30-d readmission | 101 | (37.6%) |
| 30-d mortality | 7 | 1.9% |
| 1-y mortality | 15 | 4.1% |
| Preoperative TEG | ||
| R time, s | 50 | (40–60) |
| k time, s | 120 | (76.3–180) |
| MA, mm | 52.1 | (45–59.1) |
| α angle, ° | 72.7 | (66.4–77.1) |
| Preoperative coagulation status | ||
| Hypocoagulable | 170 | (46.7%) |
| Nonhypocoagulable | 194 | (53.3%) |
DCD, donation after cardiac death; ETOH, alcoholic cirrhosis; HBV, hepatitis B virus; HCV, hepatitis C virus; NASH, nonalcoholic steatohepatitis; SLK, simultaneous liver-kidney.
Table 2.
Changes in coagulation profiles between preoperative versus postoperative
| Coagulation profile | Preoperative hypocoagulable |
Preoperative nonhypocoagulable |
||
|---|---|---|---|---|
| N | (%) | N | (%) | |
| Postoperative hypocoagulable | 67 | (55.4%) | 43 | (35.3%) |
| Postoperative nonhypocoagulable | 54 | (44.6%) | 79 | (64.8%) |
n = 243 for cohort with complete pre- and posteropative TEG data.
Table 3.
Distribution of coagulation profiles by phase of liver transplantation
|
LT phase |
Hypocoagulable |
Nonhypocoagulable |
||
|---|---|---|---|---|
| N | (%) | N | (%) | |
| Preoperative | 54 | (49.1%) | 56 | (50.9%) |
| Anhepatic | 73 | (66.4%) | 37 | (33.6%) |
| Neohepatic | 83 | (75.5%) | 27 | (24.5%) |
| Postoperative | 50 | (45.5%) | 60 | (54.5%) |
n = 110 for cohort with complete TEG data at all phases of LT.
3.2. Comparison of patients by preoperative coagulation state
Patients who were hypocoagulable by TEG assessment at the start of LT were compared to those who were nonhypocoagulable (Table 4). There were no significant differences found between the groups with regard to recipient characteristics including sex, ethnicity, BMI, or etiology of liver disease (all P > .05). Nonhypocoagulable patients were slightly older than hypocoagulable patients (59 vs 57, P = .04). Hypocoagulable patients had higher median MELD (26 vs 22, P < .01). There were no differences in the donor characteristics between the groups (all P > .05).
Table 4.
Demographics for patients undergoing LT by preoperative coagulation state
| Hypocoagulable |
Nonhypocoagulable |
P |
|||
|---|---|---|---|---|---|
| N (%)/Median (IQR) | N (%)/Median (IQR) | ||||
| Age, y | 57 | (49–62.3) | 59 | (52–64) | .036⁎ |
| Sex, male | 104 | (61.2%) | 127 | (65.5%) | .397 |
| BMI, kg/m2 | 30 | (26–34) | 30 | (25–33) | .248 |
| MELD (allocation) | 26 | (22–34) | 22 | (19–27) | < .001⁎ |
| MELD (allocation) over 35 | 32 | (18.8%) | 8 | (4.1%) | < .001⁎ |
| Ethnicity | .835 | ||||
| White | 151 | (88.2%) | 176 | (90.7%) | |
| African American | 17 | (10.0%) | 16 | (8.3%) | |
| Other | 2 | (1.2%) | 2 | (1.0%) | |
| ABO blood type | .750 | ||||
| A | 69 | (40.6%) | 70 | (36.1%) | |
| B | 17 | (10.0%) | 25 | (12.9%) | |
| AB | 10 | (5.9%) | 11 | (5.7%) | |
| O | 74 | (43.5%) | 88 | (45.4%) | |
| Etiology | .295 | ||||
| ETOH | 37 | (21.8%) | 40 | (20.6%) | |
| NASH | 34 | (20.0%) | 49 | (25.8%) | |
| HCV/HBV | 55 | (32.4%) | 55 | (28.4%) | |
| Other | 44 | (25.9%) | 50 | (25.8%) | |
| Diabetes | 39 | (22.9%) | 58 | (29.9%) | .134 |
| LT type | .064 | ||||
| Whole | 160 | (94.1%) | 171 | (88.6%) | |
| SLK | 10 | (5.9%) | 22 | (11.4%) | |
| Hypertension | 67 | (39.4%) | 97 | (50.0%) | .043⁎ |
| HCC | 30 | (17.7%) | 57 | (29.4%) | .009⁎ |
| Dialysis prior to LT | 38 | (22.4%) | 20 | (10.3%) | .002⁎ |
| Admitted at time of LT | 80 | (47.1%) | 29 | (15.0%) | < .001⁎ |
| PVT | 17 | (10.1%) | 17 | (8.7%) | .672 |
| SBP | 11 | (6.5%) | 8 | (4.1%) | .351 |
| Donor characteristics | |||||
| Donor age | 36 | (27–50) | 40 | (28–54) | .116 |
| DCD | 11 | (6.5%) | 13 | (6.7%) | .930 |
| Preoperative INR | 2.1 | (1.7–2.6) | 1.4 | (1.2–1.7) | < .001⁎ |
| Warm ischemia time | 34 | (30–40) | 34 | (30–41) | .581 |
| Total OR time | 340 | (294–405) | 336 | (293–400) | .749 |
| Preoperative TEG | |||||
| R time, s | 55 | (45–70) | 40 | (35–50) | < .001⁎ |
| k time, sec | 185 | (155–226) | 80 | (60–105) | < .001⁎ |
| MA, mm | 44.4 | (39.6–47.6) | 58.4 | (54.8–62.8) | < .001⁎ |
| α angle, ° | 65.8 | (60.8–69.6) | 76.6 | (74.1–78.6) | < .001⁎ |
HCC, hepatocellular carcinoma; OR, operating room; PVT, portal vein thrombosis; SBP, spontaneous bacterial peritonitis.
P < .05.
Outcomes between these 2 groups were then evaluated (Table 5). Hypocoagulable patients had higher transfusion requirements, requiring more pRBCs (7 vs 4 U, P < .001), FFP (14 vs 8 U, P < .001), cryoprecipitate (2 vs 1 U, P < .01), platelets (3 vs 2 U, P < .01), and cell saver (3 vs 2 L, P < .01) during the operation compared to those with nonhypocoagulable preoperative TEGs. In the first 24 hours postoperatively, a larger proportion of hypocoagulable patients were administered cryoprecipitate (25.3% vs 14.4%, P < .01) and platelets (37.1% vs 19.6%, P < .01), but postoperative transfusion of pRBCs (44.1% vs 35.1%, P = .08) and FFP (45.3% vs 36.6%, P = .09 were similar. On multivariate analysis, preoperative coagulation status was not independently associated with 24-hour transfusion requirements (data not shown).
Table 5.
Outcomes for patients undergoing LT by preoperative coagulation state
| Start hypocoagulable |
Start nonhypocoagulable |
P |
|||
|---|---|---|---|---|---|
| N (%) / Median (IQR) | N (%) / Median (IQR) | ||||
| Transfusion requirements | |||||
| pRBCs | 7 | (4–10) | 4 | (2–9) | < .001⁎ |
| FFP | 14 | (9-24) | 8 | (3.75–14) | < .001⁎ |
| Cryoprecipitate | 2 | (1–5) | 1 | (0–2) | < .001⁎ |
| Platelets | 3 | (2–5) | 2 | (0–3) | < .001⁎ |
| Cell saver | 3 | (1.75–5.1) | 2 | (1–3.33) | < .001⁎ |
| Estimated blood loss, L | 8 | (5–13.9) | 5.5 | (3–9.15) | < .001⁎ |
| 24-h pRBC | 75 | (44.1%) | 68 | (35.1%) | .077 |
| 24-h FFP | 77 | (45.3%) | 71 | (36.6%) | .092 |
| 24-h cryoprecipitate | 43 | (25.3%) | 28 | (14.4%) | .009⁎ |
| 24-h platelets | 63 | (37.1%) | 38 | (19.6%) | < .001⁎ |
| Surgical outcomes | |||||
| Reoperation | 61 | (36.1%) | 65 | (33.9%) | .656 |
| Unplanned reoperation | 36 | (21.2%) | 39 | (20.3%) | .840 |
| Open abdomen | 34 | (20.1%) | 30 | (15.6%) | .265 |
| Allograft outcomes | |||||
| HAT | 3 | (1.76%) | 7 | (3.65%) | .276 |
| Biliary complications | 28 | (16.5%) | 37 | (19.1%) | .518 |
| DGF | 10 | (5.92%) | 13 | (6.77%) | .740 |
| Hospital course | |||||
| Vent > 24 h | 61 | (36.1%) | 49 | (25.5%) | .029 |
| ICU LOS | 3 | (2–5) | 3 | (2–4) | .113 |
| ICU readmission | 21 | (12.4%) | 20 | (10.3%) | .538 |
| Hospital LOS | 9 | (6–16) | 8 | (6–14) | .121 |
| 30-d readmission | 50 | (38.2%) | 51 | (37.0%) | .838 |
| 30-d mortality | 4 | (2.4%) | 3 | (1.6%) | .710 |
| 1-y mortality | 9 | (5.3%) | 6 | (3.1%) | .306 |
DGF, delayed graft function; HAT, hepatic artery thrombosis; LOS, length of stay.
P < .05.
Despite differences in blood product utilization, no differences between groups for postoperative outcomes were detected including number of reoperations (total and unplanned), number of patients requiring temporary abdominal closure, length of stay, readmission, or mortality.
4. DISCUSSION
Herein, we studied TEG-based coagulation profiles of patients undergoing LT and found that, preoperatively, half of patients were hypocoagulable and half were nonhypocoagulable, with very few patients meeting criteria for hypercoagulable. Moreover, coagulation status was found to be dynamic, with 40% of patients finishing the operation in a different coagulation category than which they started. We identified that hypocoagulability increased during progression from the preoperative to the anhepatic and neohepatic phases of LT but normalized in the early postoperative setting. Compared to nonhypocoagulable patients, patients who were hypocoagulable preoperatively received increased perioperative administration of plasma, cryoprecipitate, platelets, and autologously transfused blood during LT, as well as increased cryoprecipitate and platelets in the first 24 hours after LT. Nonetheless, postoperative outcomes were similar between the groups.
TEG is a point-of-care hemostasis assay which can assess the mechanical properties of clot formation, progression, stability, as well as the resulting dynamics of the coagulation cascade [1,2]. Although TEG has proven itself important in other surgical fields, data on the characterization of TEG-based coagulopathy throughout LT are not well described. This study serves as the first to our knowledge to characterize specific TEG profiles during the phases of LT. We found a 26.4% increase in the prevalence of hypocoagulability from the preoperative to anhepatic state. However, this transition to a hypocoagulable state subsided following LT upon arriving to the ICU with the initiation of allograft function.
During LT, a complex array of physiological factors incites alterations of a patient's coagulation and destabilizes the prothrombotic and antithrombotic equilibrium [13]. These stressors include hemorrhage, dilutional coagulopathy, alternations in hepatic synthetic function, and changes in metabolic release and clearance of coagulation factors [10,13,14]. Initially, there is possibility for bleeding during hepatic dissection and recipient hepatectomy which can be significantly exacerbated by portal hypertension [10]. Next, during the anhepatic phase, there is a loss of synthetic function with decreased factor production and decreased tissue plasminogen activator (tPA) clearance. These changes shift the hemostatic balance toward hypocoagulation and hyperfibrinolysis [10]. Subsequently, the neohepatic phase represents a period of hemostatic vulnerability with potential for clinically relevant coagulopathies. Reperfusion may cause a heparin-like effect identifiable on TEG with a prolonged R and k time and decreased MA [15]. As the liver allograft is reperfused, an increase in tPA production during the neohepatic phase augments hyperfibrinolysis. Our work supports these pathophysiologic processes because we found an increase in prevalence of hypocoagulability throughout LT.
As TEG becomes more commonplace in surgery, including LT, it is logical then to study the impact TEG use has on transfusion requirements. Our data showing increased blood product administration in hypocoagulable patients suggest utility in correlating preoperative TEG with transfusion requirements. These findings are particularly important in the context of the increasing awareness of the negative ramifications of massive transfusions. There is no consensus on TEG-based transfusion guidelines in LT, and there is a paucity of data about the benefits of adopting such guidelines to further reduce the administration of blood products [10]. Existing work in LT is limited. Pietri et al determined significantly decreased plasma and platelet administration in LT when transfusing based on TEG compared to protocols defined by INR and serum platelet count [1]. Similarly, Wang et al identified that TEG-based transfusion in LT successfully reduced the quantity of plasma administered compared to the protocols based on prothrombin time and INR [11].
As may be expected, preoperative hypocoagulable TEG is associated with patients who have more advanced disease and significant hepatic pathology. We found that hypocoagulable patients had increased MELD scores and a higher proportion were admitted at the time of LT, most commonly to the medical intensive care unit for an exacerbation or decompensation of their underlying disease. In contrast, hepatocellular carcinoma was more common in nonhypocoagulable patients. This may be due to these patients having less advanced end-organ failure, as the liver transplant allocation system provides allocation points for patients with hepatocellular carcinoma.
In light of these patient differences, which ultimately serve as risk factors for hypercoagulation and is thus captured by their TEG profile, we noted that hypocoagulable patients had higher estimated blood loss and increased intraoperative and postoperative blood product administration. Although preoperative hypocoagulable TEG readings were associated with increased transfusion requirements, we did not demonstrate a difference in postoperative outcomes or graft function between groups. Moreover, TEG-defined hypocoagulable state was not an independent predictor of transfusion requirements in the first 24 hours postoperatively on multivariate analysis. This is likely due to the nature of these outcomes, such as length of stay and reoperation rate, which depend on multiple factors, of which blood loss and resuscitation play a part. Elevated INR has been shown to be correlated with decreased survival, but the relationship between TEG readings and the impact on long-term graft and patient outcomes has not been previously demonstrated [1]. The similarity in postoperative outcomes between groups may be attributed to the appropriate administration of intraoperative transfusions and resulting stabilization of coagulopathy once in the postoperative setting. Despite similar outcomes, the importance of our findings relates to our demonstrating the utility in using TEG for assessment of coagulation status in the hemodynamically complex OLT. Future work to understand possible predictive abilities of TEG will prove valuable.
This study has several limitations. First, the retrospective nature of the study imparts certain biases inherent to such study design. Second, regulation of fibrinolysis has been shown to be an integral component of maintaining physiologic coagulation status; however, this variable was not included in our study's definition of coagulability [16]. Third, we used previously described cutoffs for TEG values to stratify patients' coagulation status. However, well-defined TEG values among LT patients are unknown, and shifts in cutoff values could alter definitions of coagulation status. Fourth, we cannot fully account to what degree TEG itself functioned as an intraoperative evaluative tool that influenced blood product administration. The decision to administer blood products intraoperatively was determined by the anesthesiologist's and surgeon's assessment in the operating room of subjective coagulation status in conjunction with the TEG, although no formal protocols were used.
In conclusion, patients undergoing LT suffer from liver disease with imbalances in coagulation factors leading to a delicate state of fluctuation between hypo- and hypercoagulability. Using TEG-based assessment of coagulation status, we identified that a majority of patients underwent LT in a hypocoagulable state with a greater prevalence of hypocoagulability during the anhepatic and neohepatic phases, which normalizes postoperatively. When available, TEG assessment should be performed for patients undergoing LT to optimize surgical planning and improve resource utilization during LT. Moving forward, future work is required to provide insight to the additional predictive potential of TEG, as well as its impact on long-term outcomes.
Author contributions
JTG contributed to data collection, data analysis, data interpretation, and writing of the manuscript. ARC and VKD contributed to data analysis, data interpretation, and writing the manuscript. CW contributed to data collection. MCC, SAS, and MDG contributed to data interpretation and writing the manuscript. Revision, approval, and accountability of the article were carried out by all authors.
Conflict of interest
The authors have no conflicts of interest to declare.
Funding sources
This research did not receive any funding.
References
- 1.De Pietri L.B.M., Montalti R., De Maria N., Di Maira T., Begliomini B. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized. controlled trial Hepatology. 2016;63(2):566–573. doi: 10.1002/hep.28148. [DOI] [PubMed] [Google Scholar]
- 2.Stravitz R.T. Potential applications of thromboelastography in patients with acute and chronic liver disease. Gastroenterol Hepatol (N Y) 2012;8(8):513–520. [PMC free article] [PubMed] [Google Scholar]
- 3.Flores B., Trivedi H.D., Robson S.C., Bonder A. Hemostasis, bleeding and thrombosis in liver disease. Journal of translational science. 2017;3(3) doi: 10.15761/JTS.1000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caldwell S.H.H.M., Lisman T., Macik B.G., Northup P.G., Reddy K.R. Coagulation disorders and hemostasis in liver disease: pathophysiology and critical assessment of current management. Hepatology. 2006;44(4):1039–1046. doi: 10.1002/hep.21303. [DOI] [PubMed] [Google Scholar]
- 5.Zahr Eldeen F.R.G., Derosas C., Rao R., Khan M.S., Gunson B.K. Preoperative thromboelastography as a sensitive tool predicting those at risk of developing early hepatic artery thrombosis after adult liver transplantation. Transplantation. 2016;100(11):2382–2390. doi: 10.1097/TP.0000000000001395. [DOI] [PubMed] [Google Scholar]
- 6.Park MS MW, Dubick MA, Salinas J, Butenas S, Kheirabadi BS, et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009;67(2):266–275; discussion 275–266. [DOI] [PMC free article] [PubMed]
- 7.Plotkin A.J.W.C., Jenkins D.H., Smith K.A., Noe J.C., Park M.S. A reduction in clot formation rate and strength assessed by thrombelastography is indicative of transfusion requirements in patients with penetrating injuries. J Trauma. 2008;64(2 Suppl):S64–S68. doi: 10.1097/TA.0b013e318160772d. [DOI] [PubMed] [Google Scholar]
- 8.Kang Y. Transfusion based on clinical coagulation monitoring does reduce hemorrhage during liver transplantation. Liver Transpl Surg. 1997;3(6):655–659. doi: 10.1002/lt.500030621. [DOI] [PubMed] [Google Scholar]
- 9.Tripodi A.P.M., Chantarangkul V., Viscardi Y., Dell’Era A., Fabris F.M., Mannucci P.M. The coagulopathy of cirrhosis assessed by thromboelastometry and its correlation with conventional coagulation parameters. Thromb Res. 2009;124(1):132–136. doi: 10.1016/j.thromres.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Clevenger B., Mallett S.V. Transfusion and coagulation management in liver transplantation. World J Gastroenterol. 2014;20(20):6146–6158. doi: 10.3748/wjg.v20.i20.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S.C.S.J., Chang K.Y., Chu Y.C., Liu C.S., Loong C.C. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplant Proc. 2010;42(7):2590–2593. doi: 10.1016/j.transproceed.2010.05.144. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann C.R., Dwyer K.M., Crews J.D., Dols S.J., Trask A.L. Usefulness of thrombelastography in assessment of trauma patient coagulation. J Trauma. 1997;42(4):716–720. doi: 10.1097/00005373-199704000-00023. [discussion 720-712] [DOI] [PubMed] [Google Scholar]
- 13.Verbeek T.A., Stine J.G., Saner F.H., Bezinover D. Hypercoagulability in end-stage liver disease: review of epidemiology, etiology. and Management Transplantation direct. 2018;4(11):e403. doi: 10.1097/TXD.0000000000000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Pietri L., Montalti R., Nicolini D., Troisi R.I., Moccheggiani F., Vivarelli M. Perioperative thromboprophylaxis in liver transplant patients. World J Gastroenterol. 2018;24(27):2931–2948. doi: 10.3748/wjg.v24.i27.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal S., Senzolo M., Melikian C., Burroughs A., Mallett S.V. The prevalence of a heparin-like effect shown on the thromboelastograph in patients undergoing liver transplantation. Liver Transpl. 2008;14(6):855–860. doi: 10.1002/lt.21437. [DOI] [PubMed] [Google Scholar]
- 16.Porte R.J., Bontempo F.A., Knot E.A., Lewis J.H., Kang Y.G., Starzl T.E. Systemic effects of tissue plasminogen activator- associated fibrinolysis and its relation to thrombin generation in orthotopic liver transplantation. Transplantation. 1989;47(6):978. doi: 10.1097/00007890-198906000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]