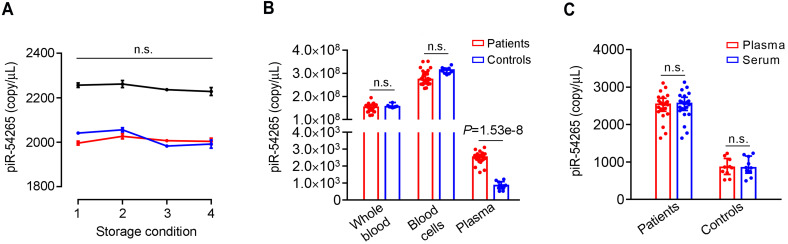
Figure 1.
Serum piR-54265 is stable and feasible in both serum and plasma for detection and quantification. A, The piR-54265 level in serum of 3 randomly selected individuals with CRC under different storage conditions. Storage conditions were: 1, fresh preparation at room temperature; 2, storage at 37 °C for 24 h; 3, storage at room temperature for 72 h; 4, after 3 times of repeated freeze-and-thaw. Data represent mean ± SEM of 3 measurements of each storage condition; n.s., not significant by one-way ANOVA test. B, Different piR-54265 levels in whole blood samples, blood cells and plasma of CRC cases (N = 24) and CRC-free controls (N = 10). Data represent median with inter-quartile ranges. Mann-Whitney U test shows significant difference only in plasma between CRC patients and controls; n.s., not significant. C, Plasma or serum piR-54265 levels (median with inter-quartile range) in CRC patients and controls; n.s., not significant by Mann-Whitney U test.