Figure 3.
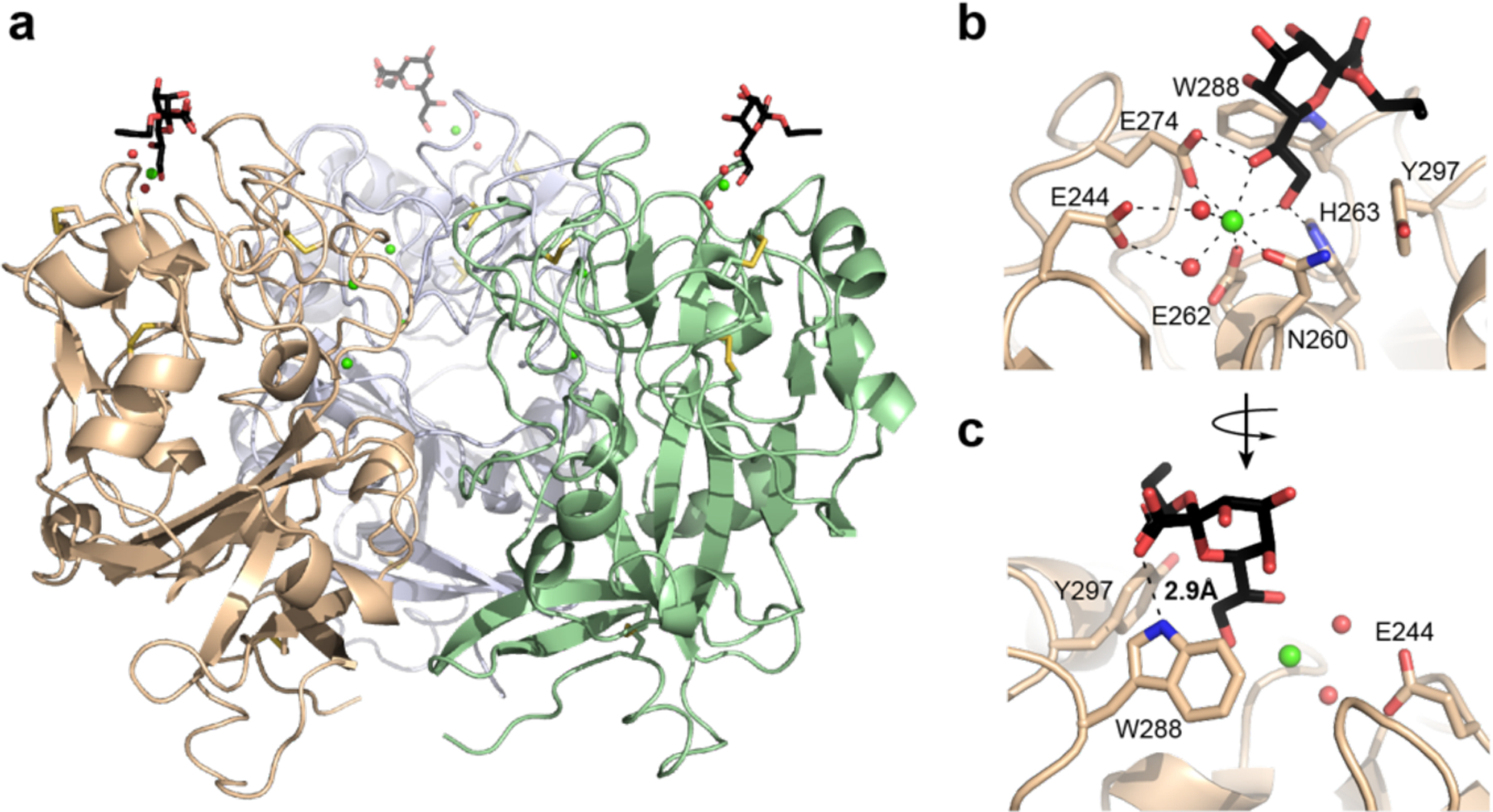
Structure of hItln-1 bound to allyl-α-KO. (a) Complex of hItln-1 trimer and allyl-α-KO. The lectin monomers are depicted in green, wheat, or light blue; the allyl-α-KO in black; calcium ions in green; intra-monomer disulfides in yellow; and ordered water molecules in the binding site in red. The trimeric structure is produced from chain A in the asymmetric unit by a three-fold crystallographic operation. (b) The carbohydrate-binding site of hItln-1 with allyl-α-KO bound. Residues involved in calcium ion coordination and ligand binding are noted. Dashed lines show heptavalent coordination of the calcium ion. (c) Rotation of the binding pocket shows a hydrogen bond between KO and W288, depicted by a dashed line (rNH…o = 2.9 Å).
