Figure 4.
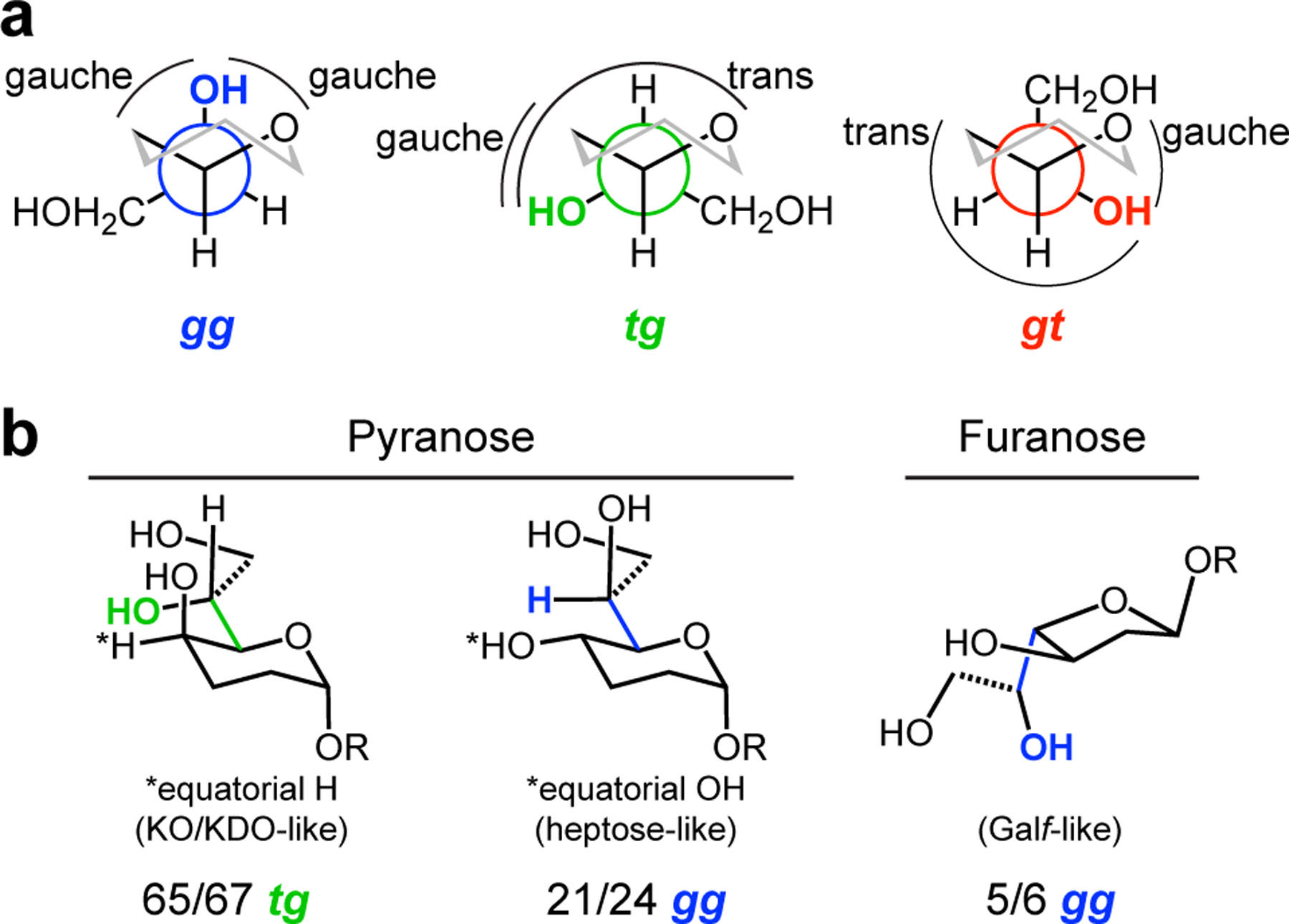
Bioinformatic analysis of exocyclic vicinal diol-containing glycans in the PDB. (a) Newman projections showing the gg, tg, and gt conformations of the proximal hydroxyl of the exocyclic vicinal diol (highlighted in blue, green or red) with respect to the C5–O bond, first, and the C5–C4 bond second (cf, C4–O and C4–C3 for furanoses). (b) The most prevalent proximal hydroxyl conformation of the three classes of carbohydrate in our analysis are shown. For pyranoses with an equatorial hydrogen, 65/67 structures are tg; pyranoses with an equatorial hydroxyl, 21/24 structures are gg; and furanoses with an equatorial hydroxyl, 5/6 structures are gg.
