Figure 5.
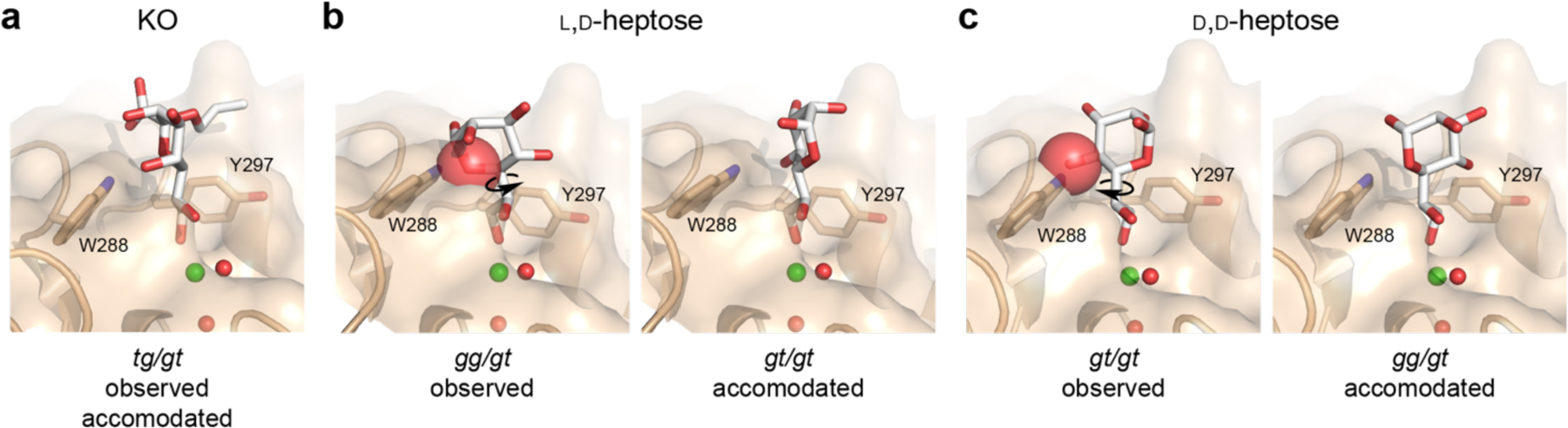
Observed and accommodated ligand conformations in hItln-1 binding site. (a) KO can bind to hItln-1 in the tg/tg conformation without steric interactions, as observed in the structure of the complex (Figure 3). Alignment of the exocyclic vicinal diol of l,d-heptose (b) or d,d-heptose (c) affords steric interaction between each ligand and W288, depicted by red spheres. Rotation about the proximal bond of the exocyclic vicinal diol side chain gives rise to ligand conformations accommodated by the hItln-1 binding site without steric interaction. Ligands are shown in white sticks, and red spheres represent the van der Waals radii of atoms with significant interactions. Observed conformations of l,d-heptose and d,d-heptose were extracted from PBD: 2rib and 2ria, respectively.
