Figure 6.
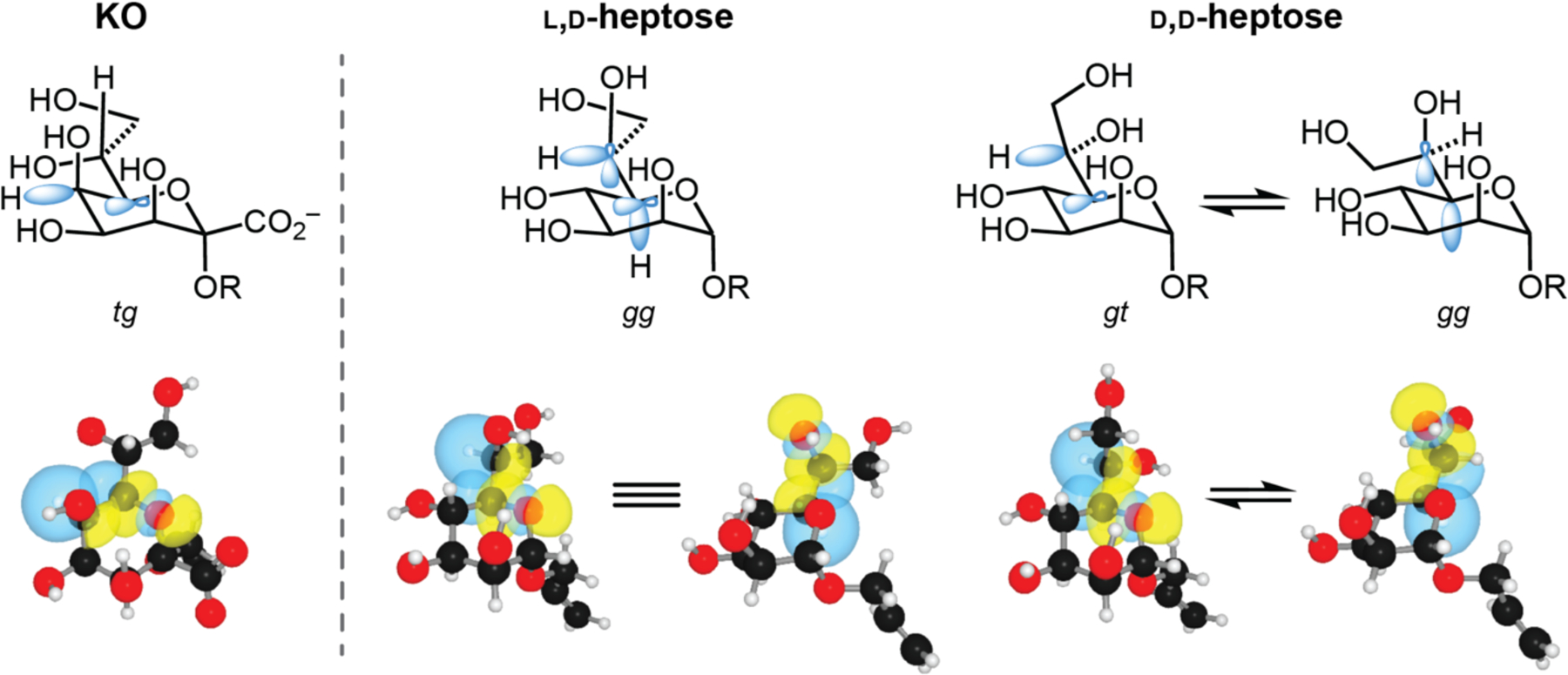
Stabilizing stereoelectronic effects of preferred rotamers of the proximal side chain C–C bond of KO, l,d-heptose, and d,d-heptose. Preferred rotamers are based on predominant conformations in published PDB structures. Relevant orbitals involved in stabilizing these conformations are represented in blue (top). NBO renderings of significant σC–H→σ*C–O interactions are depicted with blue and yellow orbitals (bottom). Rotamers shown were atom-optimized at the M06–2X/6–311+G(d,p) level of theory employing the IEFPCM solvation model. For clarity, two separate renderings are shown for each stabilizing interaction in the preferred gg l,d-heptose conformer. Comparisons of these interactions aid in explaining the differences in affinity of each monosaccharide to hItln-1.
